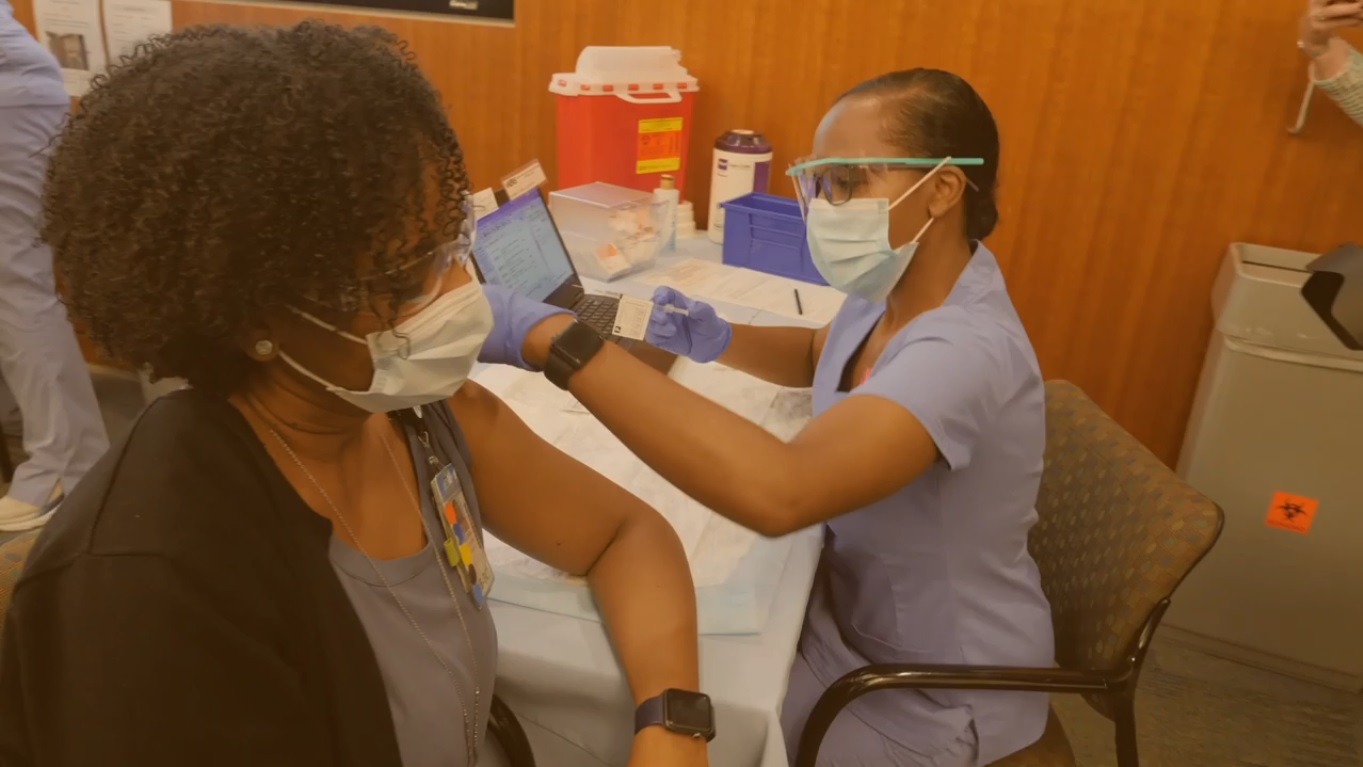
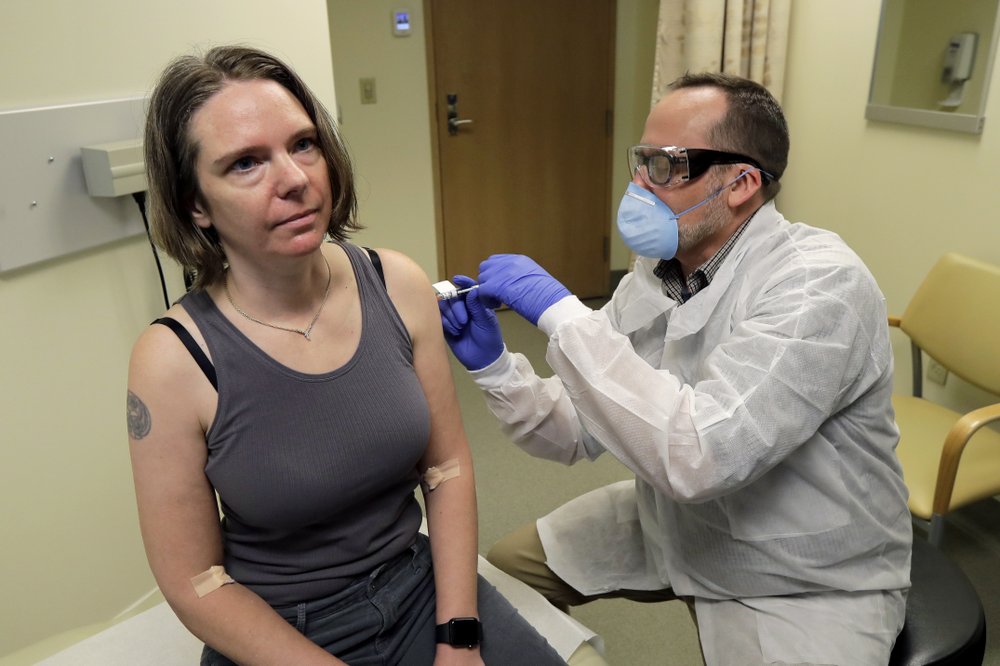
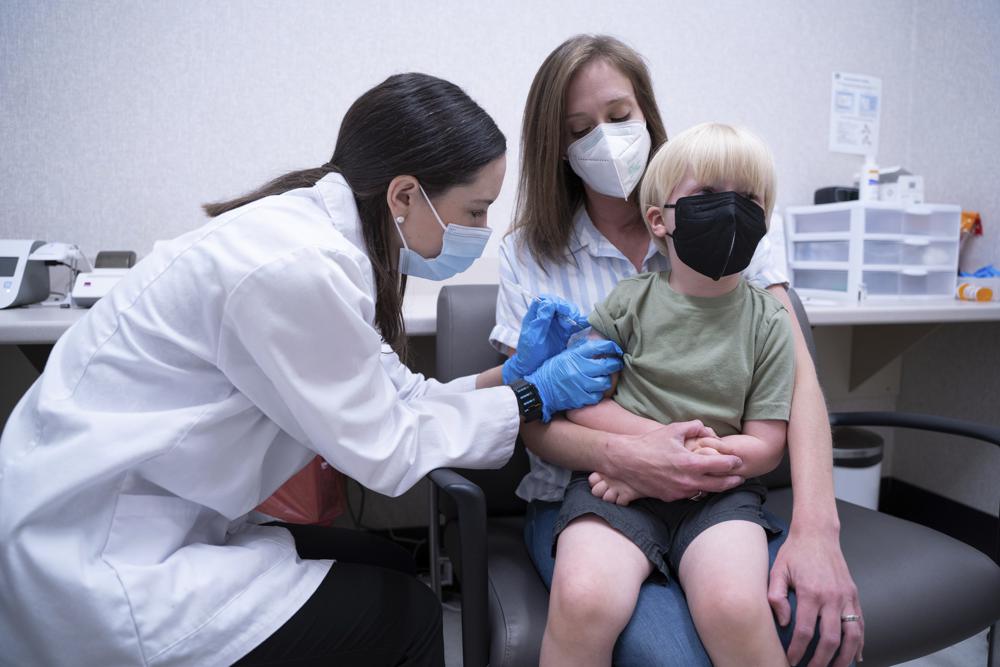
Several COVID-19 vaccine candidates, including one stemming from ongoing trials at UNC, are working to gain approval from the U.S. Food and Drug Administration (FDA).
Pfizer, the New York pharmaceutical company producing the front-running vaccine candidate, said it will ask the FDA for authorization later this month.
The FDA said COVID-19 vaccines must work at least half the time to win approval – however, early signs indicate they will be much more effective than that. In its first release of data, the candidate vaccine made by Pfizer proved 90 percent effective against the virus.
The coronavirus shots, made by Pfizer and its German partner BioNTech, are among 10 possible vaccine candidates in late-stage testing around the world. While Pfizer is on the brink of having their vaccine approved by the FDA, they are not the only ones.
Moderna Inc. said it also hopes to file an application with the FDA later this month.
At the onset of the pandemic, infectious disease experts at Carolina and across the country joined together to form the COVID Prevention Network. This network coordinates multiple studies, including the current phase 3 clinical trial for Moderna’s COVID-19 vaccine. UNC is just one of more than 90 sites testing the vaccine across the U.S.
All sites for Moderna’s phase 3 clinical trial stopped recruiting participants in October.
Chapel Hill resident Graice Howell is just one of the 174 participants recruited at UNC’s site. After losing her corporate job in hospitality due to COVID, Howell become an essential worker at Wegmans. Now, she is doing her part to help in these vaccine trials.
“It became clear to me very early in this situation that a vaccine was going to be the only way out for the world,” Howell said.
Phase 3 trials confirm and expand on phase 1 and 2 trials by evaluating a large number of people – ideally those who are at an increased risk for COVID-19 – so that the effectiveness of the vaccine can be determined.
These at-risk populations could include the Latinx community, people of color, the elderly, those with underlying health conditions and essential workers like Howell.
“The more people that join in and the more people that also feel that they can take a part in moving the ball towards the goal post, the faster we can get to the other side,” Howell said.
Much like other COVID-19 vaccines currently undergoing trials, the Moderna vaccine involves two injections separated by about one month. Howell said her experience with the phase 3 trials has not only felt safe and educational, but hopeful as we wait for further scientific advances.
“I’ve not had any painful experiences,” Howell said. “Maybe the nasal swab was not one of the greatest experiences but I want so much for this vaccine, or a vaccine, to succeed as quickly as possible. So, I’m so committed to making that happen in any way I can.”
As clinical trials continue, and the promise of FDA approval lingers on the horizon, health officials are optimistic that a vaccine will be made available at the beginning of next year.
Dr. Anthony Fauci said once a vaccine is approved – if the majority of the country can be convinced to take it – the U.S. could potentially regain a sense of normalcy by the end 2021.
Lead photo courtesy of University of Maryland School of Medicine via AP.
Chapelboro.com does not charge subscription fees. You can support local journalism and our mission to serve the community. Contribute today – every single dollar matters.