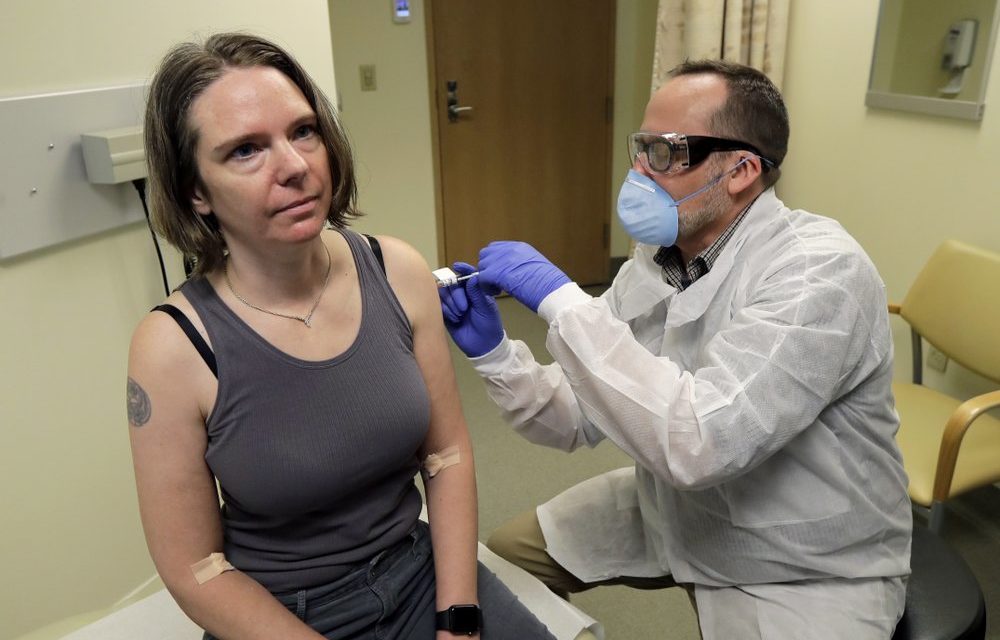
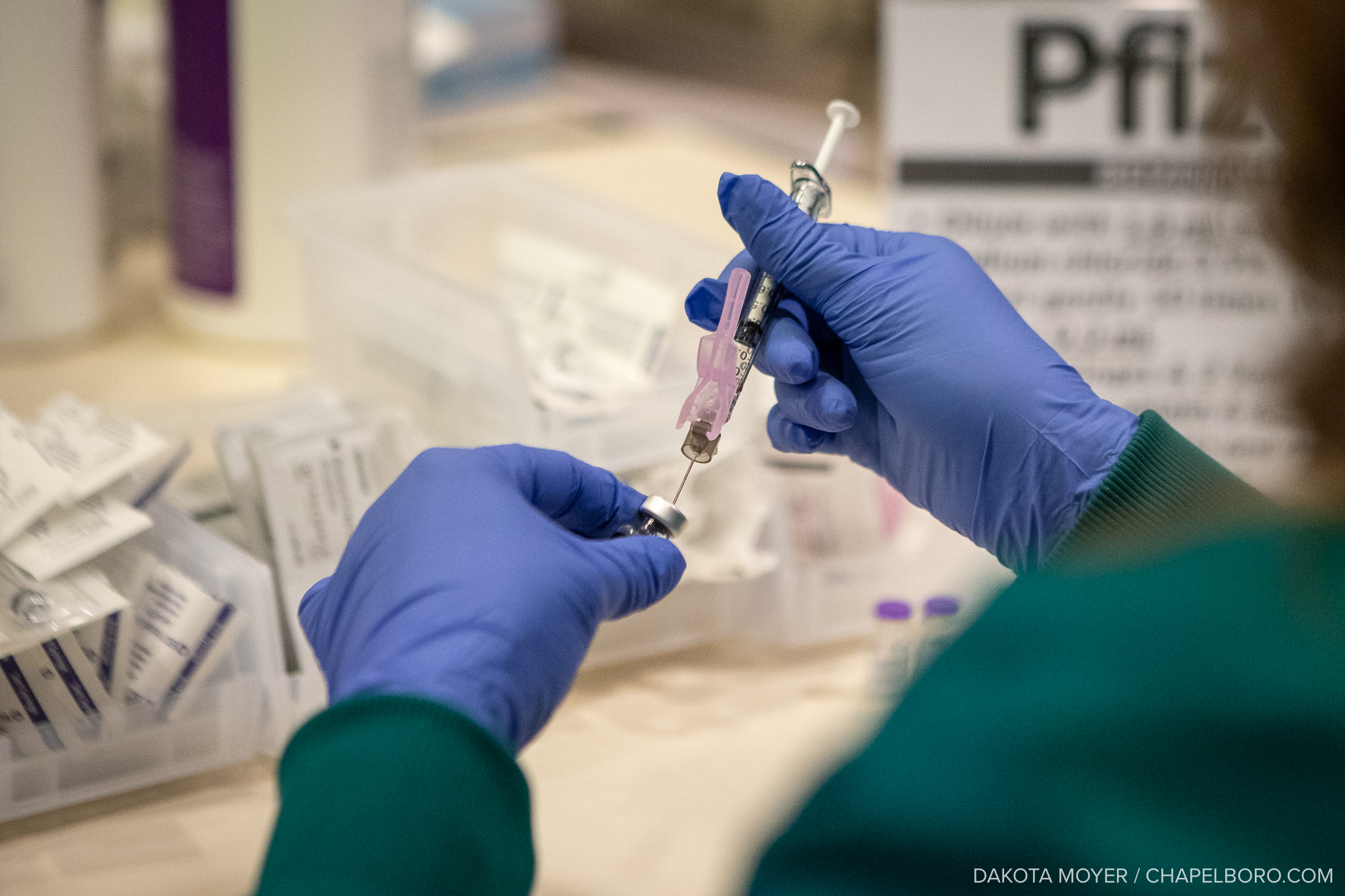
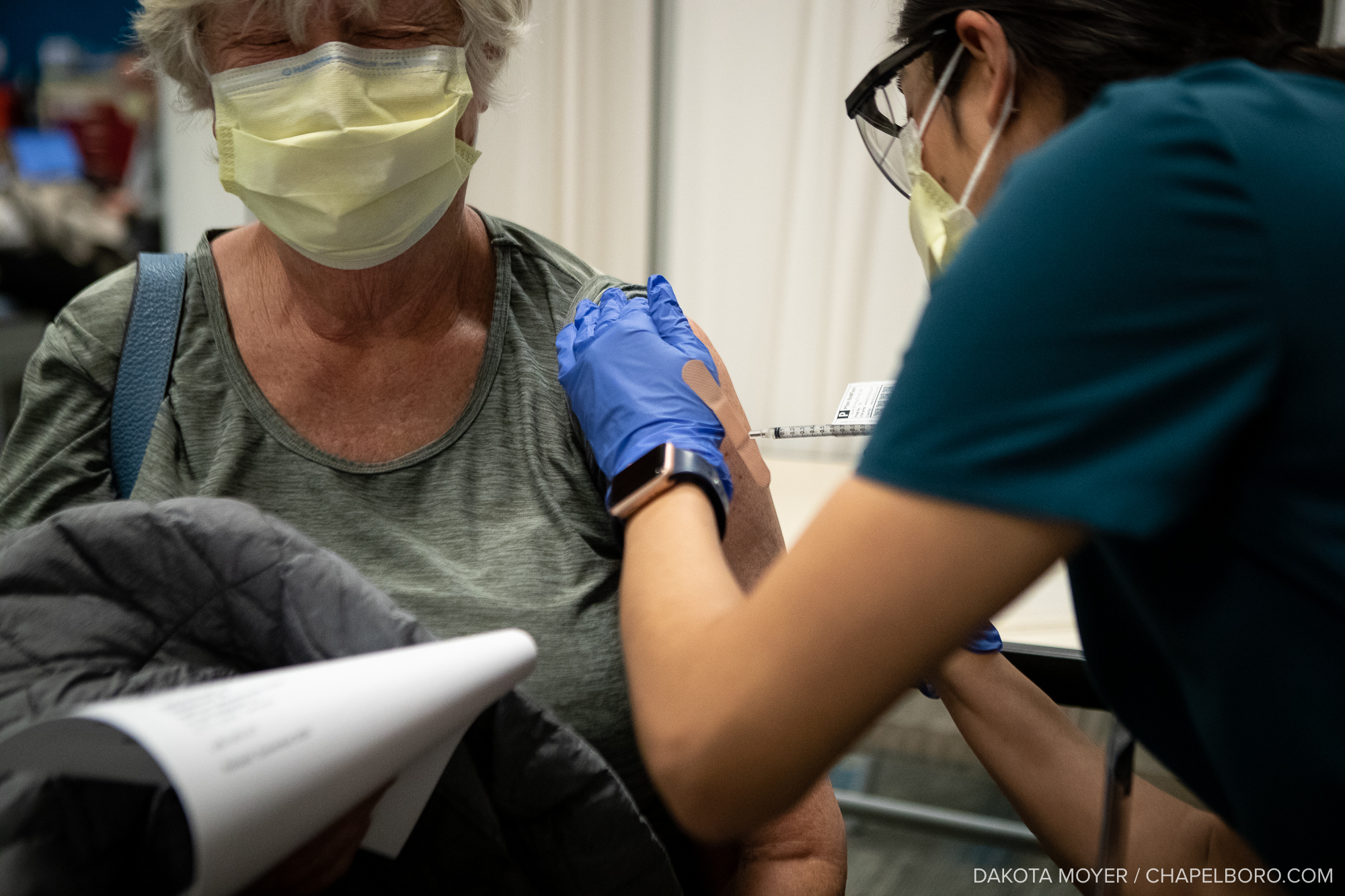
Clinical researchers in the UNC Division of Infectious Diseases are asking the community to partake in a large phase 3 clinical trial for a COVID-19 vaccine.
Sites across the country, including one at UNC, are testing the mRNA-1273 vaccine developed by Moderna in a large-scale study aiming for 30,000 participants. The potential Moderna vaccine is one of a handful of treatment and prevention studies launching soon to fight COVID-19.
Phase 3 trials confirm and expand on Phase 1 and 2 trials by evaluating a large number of people – in this case ideally those who are at an increased risk for COVID-19 – so that the effectiveness of the vaccine can be determined.
Public enrollment to be a part of the study is expected to begin next week.
Dr. Cynthia Gay is the Principle Investigator for UNC – leading the study evaluating the Moderna COVID-19 vaccine.
Gay said she and her colleagues are excited about this local research, because if this vaccine is found to be effective, it will quickly and directly impact the community.
Typically, the development of a vaccine from phase 1 to phase 3 would be more spread out, but because of the very urgent need nationwide, Gay said her team has been working double time – but she said that doesn’t mean this trial isn’t safe.
“So it’s not that steps are being skipped, it’s not that any of the normal review [is being skipped], it’s all happening, we’re just asking people to do it quicker and put other stuff on hold and to prioritize,” Gay said.
This trail vaccine study is being conducted across approximately 89 sites in the U.S. For the UNC-specific site, Gay said they are hoping to enroll 500 participants. She said there is a relatively short time to enroll in the study across the board, so Gay’s team at UNC is trying to find as many interested people as soon as possible.
She said they are particularly looking for participants whose communities have been hard hit by the pandemic.
“The reason for that is perhaps obvious, but it’s for those who are at risk to have a chance to benefit from the vaccine if it’s effective and also benefit those around them including their families and their larger communities,” Gay said.
Ideal participants could include the Latinx community, people of color, the elderly and those with underlying health conditions.
Dr. David Wohl is one of UNC’s infectious disease experts who is helping Gay test several treatments for COVID-19. Wohl said even if they develop a good vaccine, there will still be people who get infected and need treatment, which means other trials will be needed.
“At the same time that we’re launching this first of maybe several vaccine trials until we find the right vaccine, we are also launching a clinical trial of treatment,” Wohl said.
The National Institutes of Allergies and Infectious Diseases created the COVID Prevention Network to coordinate these multiple studies.
Wohl said there are exciting treatments out there right now and UNC is excited to start treatment trials alongside vaccine trials – but first and foremost they need individuals who are ready and able to participate in this research.
“We’re spending a lot of time and effort to make sure that the people we enroll in these studies reflect the pandemic here in North Carolina,” Wohl said. “So we want to make sure that those who have been heavy hit because of their essential work, because of their living situations, are also clearly represented appropriately in our studies.”
UNC has been preparing this research trial for implementation for the past six weeks. Wohl said UNC and its various research teams have had an ‘abundance of caution’ preparing for these trials – and now that they’re ready, they need help.
“We need help,” Wohl said. “We need people to literally roll up their sleeves and help us get back to normal. We’re not going to get back to normal if we’re all spectators and if there’s not folks who are willing to step up and say ‘I’m willing to be involved in that study.’”
Potential candidates can sign up to be screened for the study through a national registry, where their degree of risk for acquiring COVID-19 is assessed. For anyone interested in participating in the Moderna vaccine clinical trial, click here.
(Lead photo via Associated Press News/Ted S. Warren)
Chapelboro.com does not charge subscription fees. You can support local journalism and our mission to serve the community. Contribute today – every single dollar matters.