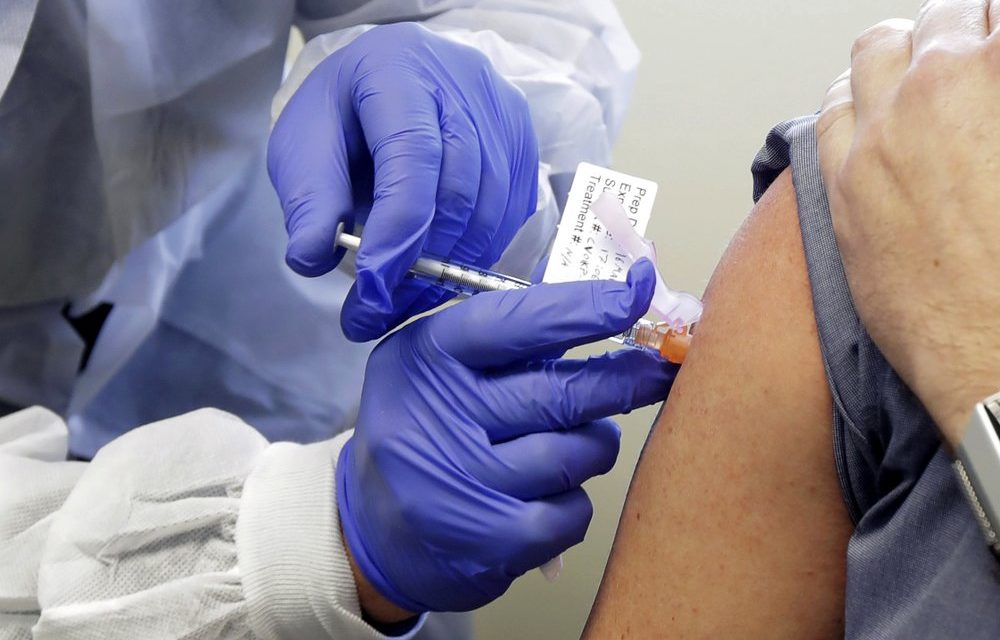
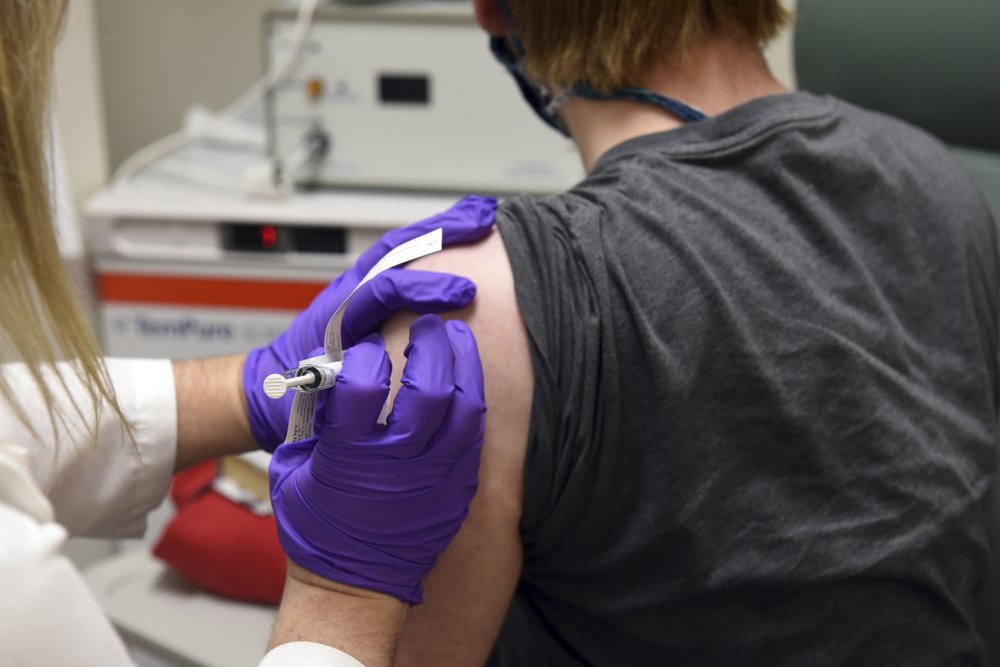
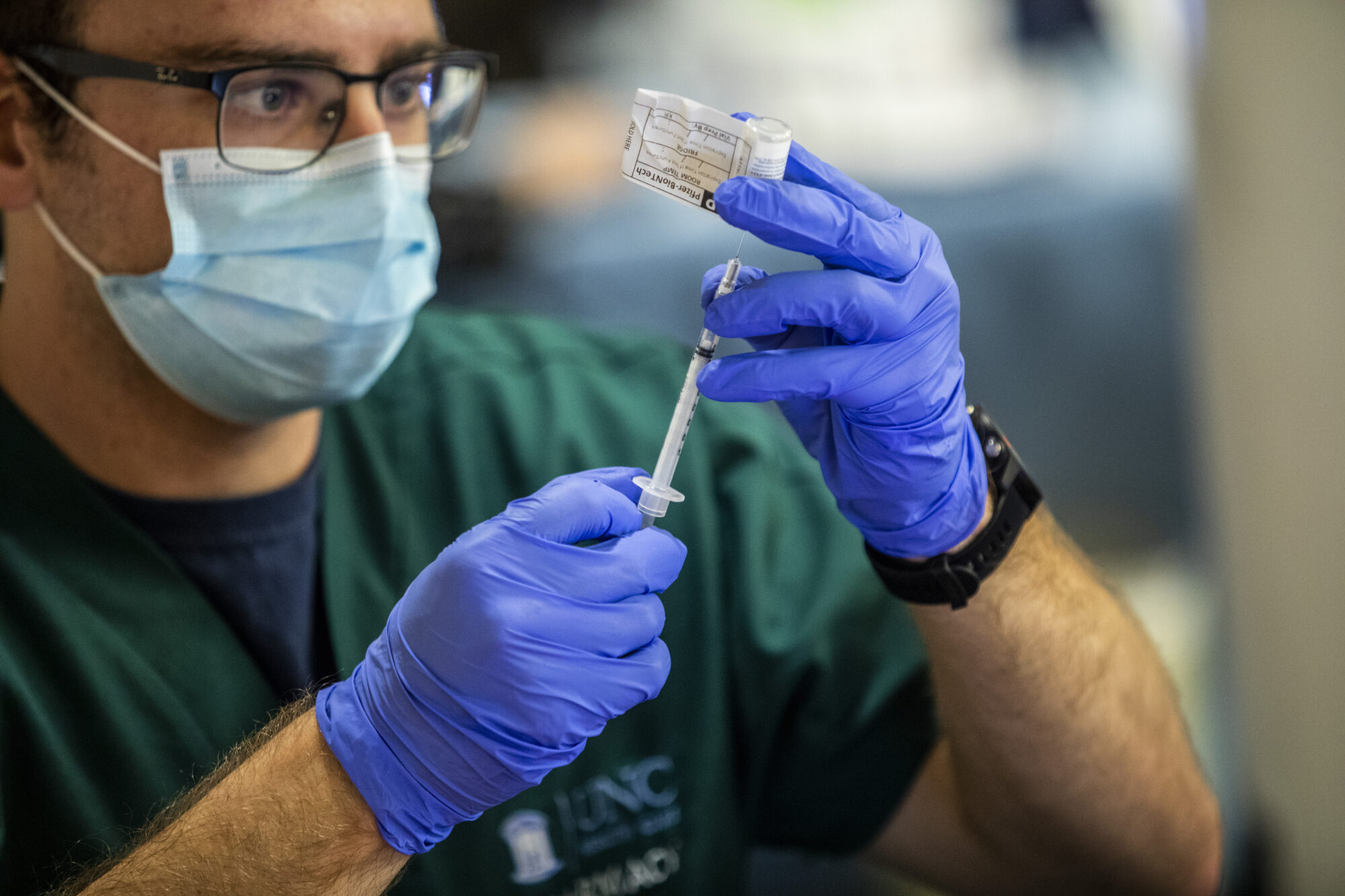
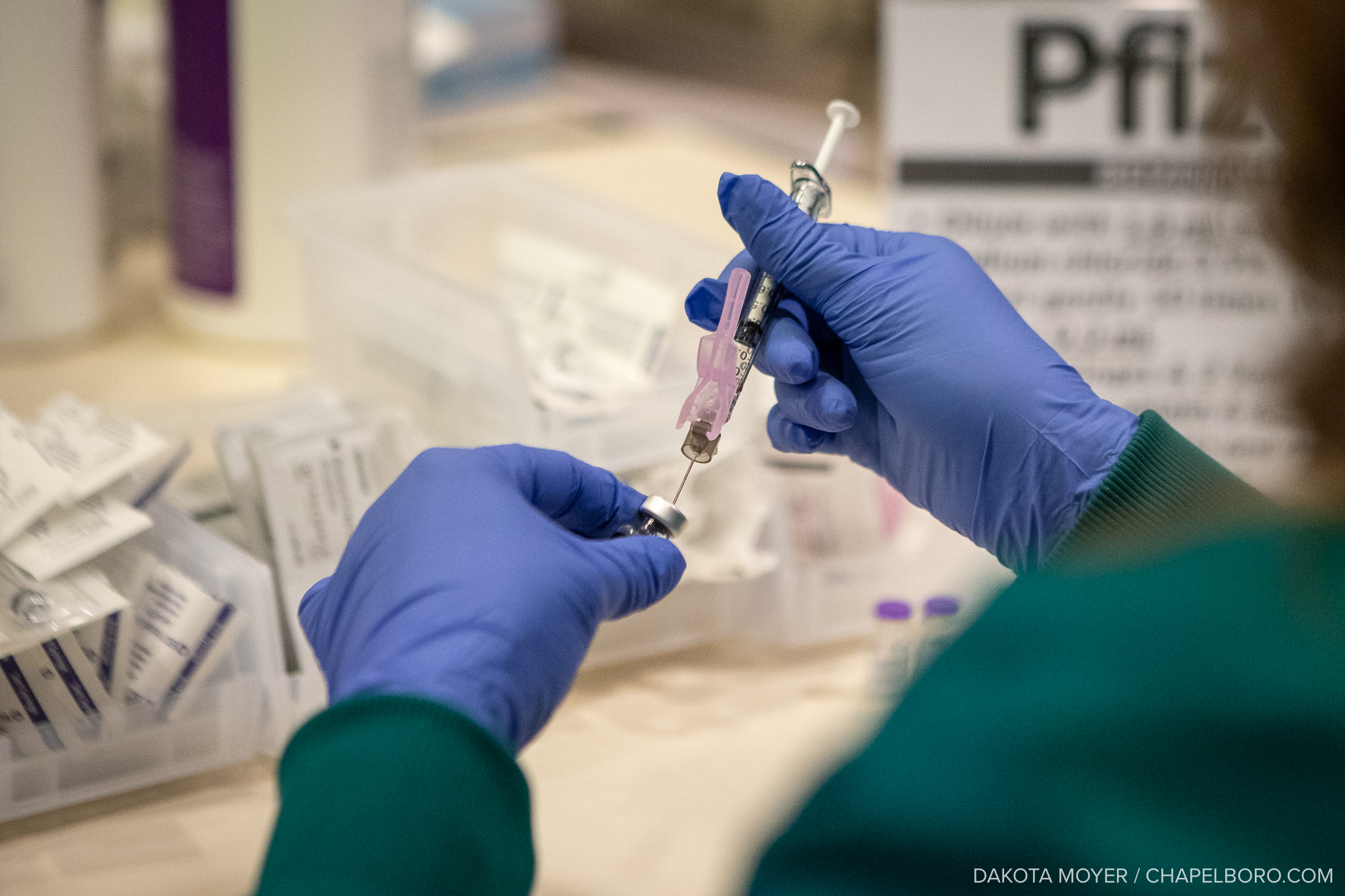
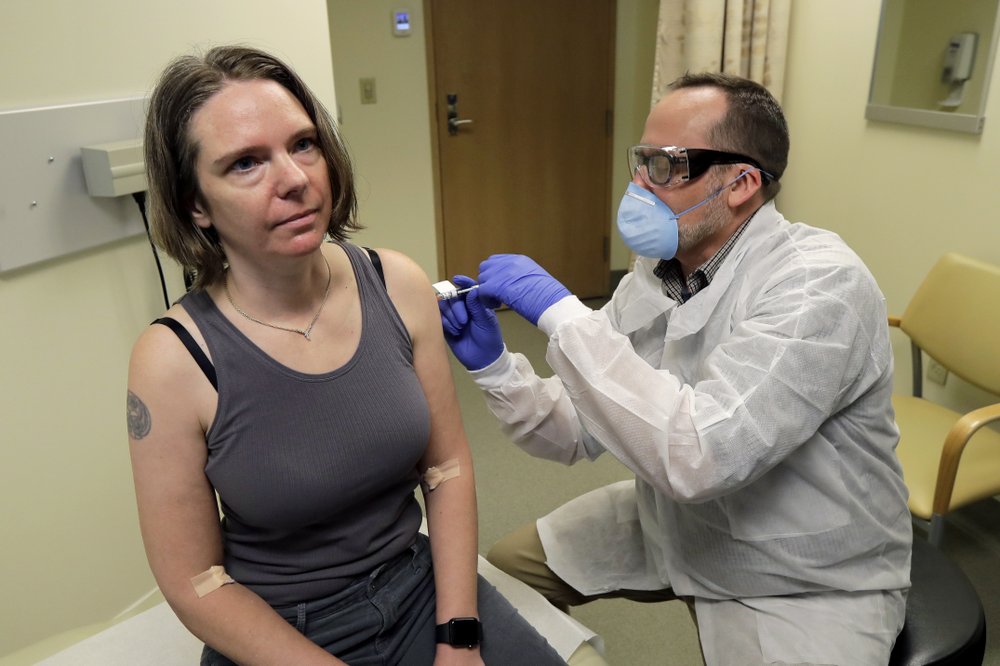
For the second week in a row, a major drug manufacturer has issued data indicating that its new vaccine could be successful at fighting COVID-19.
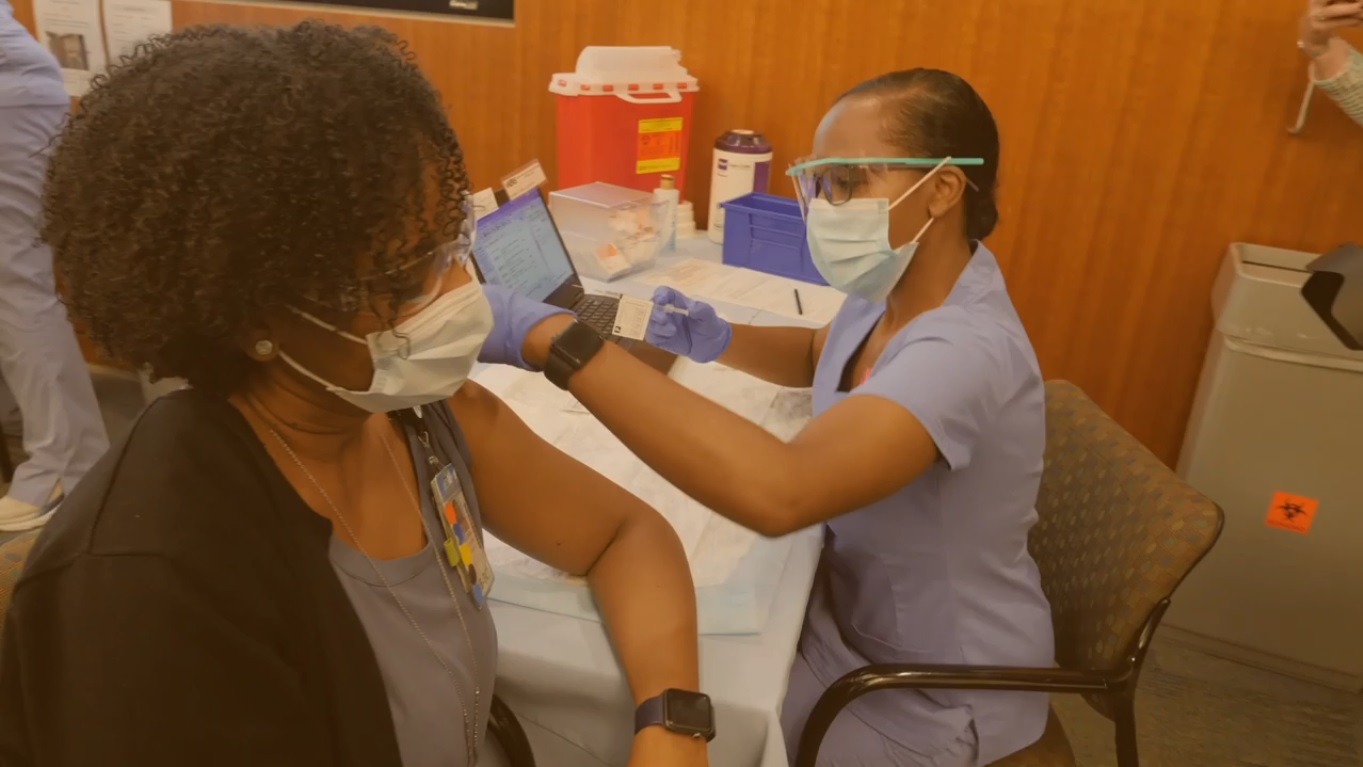
At the onset of the pandemic, infectious disease experts at Carolina and across the country joined together to form the COVID Prevention Network. This network coordinates multiple studies, including the current phase 3 clinical trial for Moderna’s COVID-19 vaccine. UNC is just one of more than 100 sites testing the vaccine across the U.S. since the study launched on July 27.
Now, after months of waiting, Moderna said its vaccine is proving to be highly effective.
In a press release on Monday, Moderna said its COVID-19 vaccine is 94.5 percent effective in its ongoing COVID-19 study. This study, known as the COVE study, was designed in collaboration with the FDA and NIH to evaluate Americans who are at-risk of severe complications from COVID-19.
Moderna’s vaccine is being studied in 30,000 volunteers who received two doses of either the trial vaccine or a dummy shot. On Sunday, 95 new infections were recorded after volunteers’ second shot – however, only five of the illnesses occurred among people given the real vaccine.
A week ago, Pfizer Inc. announced its own vaccine appeared similarly effective — news that puts both companies on track to seek permission within weeks for emergency use in the U.S. If the FDA allows emergency use of Moderna’s or Pfizer’s vaccine candidate, there will be limited, rationed supplies before the end of the year.
Both vaccines require people to get two shots, several weeks apart. According to the Associated Press, U.S. officials said they hope to have about 20 million Moderna doses and another 20 million doses of the vaccine made by Pfizer and its German partner BioNTech to use in late December.
Back in June, scientists and researchers at UNC agreed to join Moderna’s phase 3 clinical trials for a potential COVID-19 vaccine. In August, clinical researchers in the UNC Division of Infectious Diseases asked the community to partake in said trials. Out of the 30,000 participants now enrolled nationwide, 174 participants were recruited at UNC’s site.
Our team has been leading a Moderna vaccine trial at UNC this fall. Hope they can take a moment to celebrate over ☕️ before they launch a new, simultaneous trial. @PreventCOVID_19 @UNC_Health_Care @UNC_SOM https://t.co/d4tO3CUYel
— Institute for Global Health & Infectious Diseases (@uncglobalhealth) November 16, 2020
Dr. Cynthia Gay is the Principle Investigator for UNC – leading the study evaluating the Moderna COVID-19 vaccine. She said while these are hopeful and promising results, UNC’s study will remain ongoing. According to UNC Health, the Moderna phase 3 vaccine clinical trial will last two years from start to finish.
“I don’t think any of us were expecting to have such a great efficacy rate in these first studies,” Gay said. “This is more than we were hoping for. But this news is based on preliminary data, and we do need to continue with the study.
The vaccine being tested in this study is called mRNA-1273. The study is evaluating if the vaccine can help the immune system produce effective antibodies against the SARS-CoV-2 virus so that, in case of infection, the virus does not cause illness.
According to Moderna’s website, the vaccine cannot cause infection or make someone sick with COVID-19 as it is not made from the SARS-CoV-2 Virus. Typical vaccines for viruses are made from a weakened or inactive virus, but mRNA-1273 is different. Moderna said it is made from messenger ribonucleic acid (mRNA), a genetic code that tells cells how to make protein, which help the body’s immune system make antibodies to fight the virus.
To read Moderna’s first interim analysis of the phase 3 COVE study, click here.
Lead photo via AP/Ted S. Warren.
Chapelboro.com does not charge subscription fees. You can support local journalism and our mission to serve the community. Contribute today – every single dollar matters.