The U.S. Food and Drug Administration on Monday granted full approval to the Pfizer COVID-19 vaccine for people age 16 and older. The vaccine made by Pfizer and its partner BioNTech now carries the strongest endorsement from the FDA, which experts say will make it easier for businesses and governments to move forward with vaccine mandates.
Since receiving Emergency Use Authorization from the FDA in December, more than 200 million Pfizer doses have already been administered in the U.S.
The FDA initially allowed emergency use of Pfizer’s vaccine based on a study that tracked 44,000 people 16 and older for at least two months — the time period when serious side effects typically arise. As that’s shorter than the six months of safety data normally required for full approval, Pfizer kept that study going.
In the FDA’s review for approval, scientists evaluated “hundreds of thousands of pages” of vaccine data from the 40,000 trial participants, according to the U.S. agency. Based on clinical trial results, the vaccine was found to be 91 percent effective in preventing COVID-19 – slightly lower than the 95 percent efficacy rate trial data showed when the shot was authorized late last year, before the delta variant took hold in the U.S.
“It’s really good news that that effectiveness data is holding up as well as it did,” said Dr. Mark McClellan, a Duke University health policy expert and former FDA commissioner. “On the safety side, the trials of tens of thousands of people found no evidence of serious long-term side effects and no side effects that showed up after the first hours to days to a few weeks after the vaccines.”
McClellan said this full approval could help alleviate concerns for those who are vaccine hesitant. It could also help businesses, schools and states enforce their own vaccine mandates. He said such mandates may help quell the rising number of COVID cases, especially in states that are experiencing a lack of available ICU beds.
“We’ve already seen announcements today from places like the city of New York, the Pentagon for the U.S. military, and additional schools likely to take similar steps in the coming days,” McClellan said. “So those things are going to bump up our vaccination numbers.”
In Orange County, individual businesses – such as Cat’s Cradle in Carrboro, Market & Moss in Chapel Hill, and Yonder in Hillsborough – have already announced that patrons must show proof of vaccination or a negative COVID test before entry.
The U.S. government’s top infectious disease expert, Dr. Anthony Fauci, said earlier this month that full FDA approval of a COVID vaccine will spur a wave of vaccine mandates in the private sector as well as schools and universities.
“You’re going to see the empowerment of local enterprises, giving mandates that could be colleges, universities, places of business, a whole variety and I strongly support that,” said Fauci. “The time has come. We’ve got to go the extra step to get people vaccinated.”
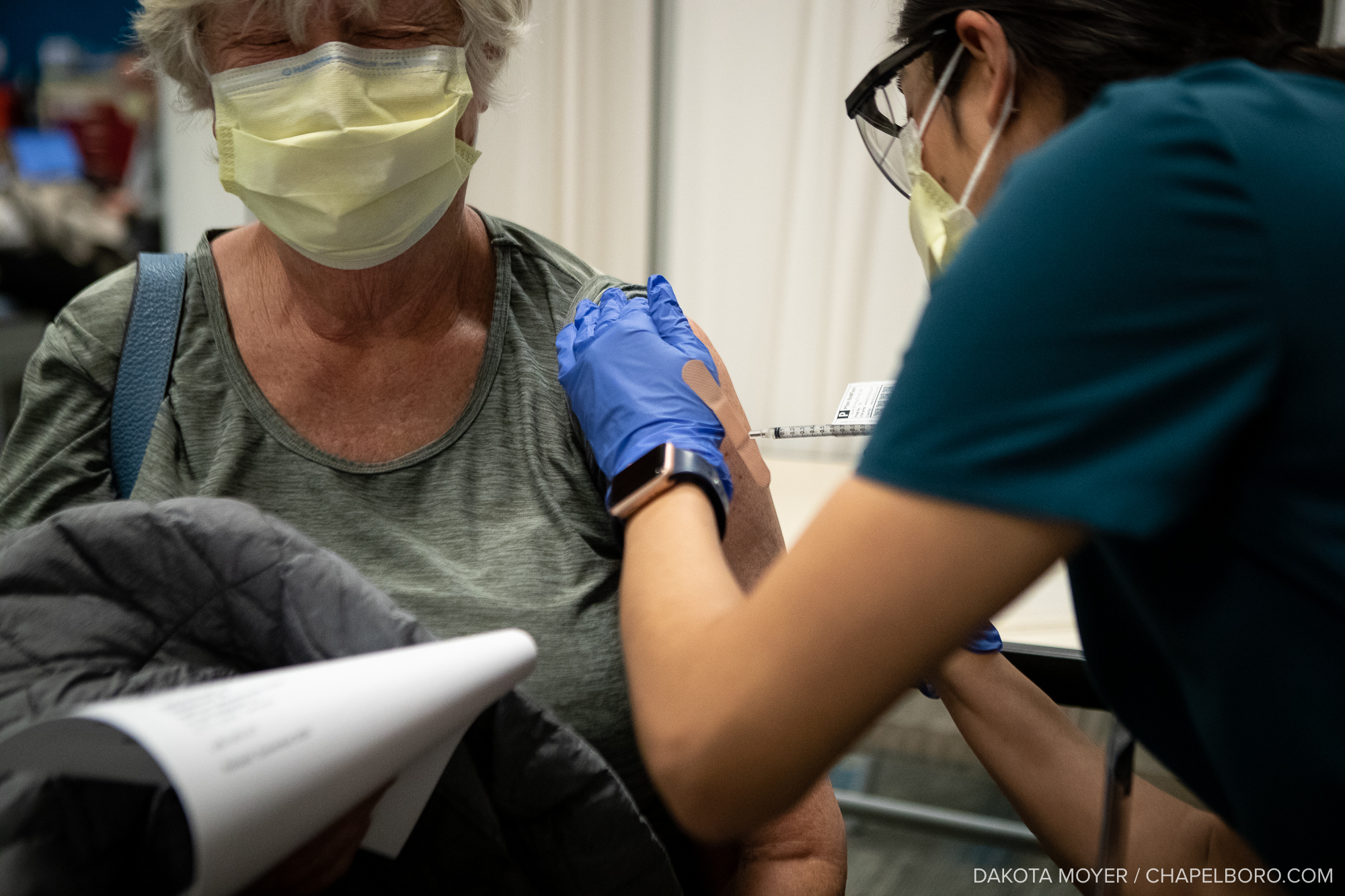
While U.S. vaccinations bottomed out in July, as the delta variant fills hospital beds, shots are on the rise again. According to the CDC, a million COVID-19 vaccines were administered this past Thursday, Friday, and Saturday respectively.
Currently, more than half of the total U.S. population, or about 170 million people, have been fully vaccinated. In North Carolina, 48 percent of the population is fully vaccinated.
Although North Carolina falls slightly behind the national average, locally, Orange County continues to set the bar for state vaccination rates with 76 percent of the county’s population fully vaccinated.
McClellan said he hopes this full FDA approval will encourage even more North Carolinians and Americans to get their vaccine.
“I think for the most part, the most important benefit, is that confidence that the FDA has applied its usual, non-emergency gold standard review process to this vaccine,” McClellan said.
In a statement from the FDA on Monday, officials said, “The public and medical community can be confident that although we approved this vaccine expeditiously, it was fully in keeping with our existing high standards for vaccines in the U.S.”
Emergency authorization for Pfizer’s vaccine was expanded to include those 12 through 15 years of age on May 10. Pfizer, alongside Duke and UNC experts, said it expects to have vaccine trial data on children ages 5 to 11 by the end of September, with Emergency Use Authorization from the FDA expected by the end of the calendar year.
Lead photo via Liam McBurney/AP.
Chapelboro.com does not charge subscription fees. You can support local journalism and our mission to serve the community. Contribute today – every single dollar matters.