To reflect on the year, Chapelboro.com is re-publishing some of the top stories that impacted and defined our community’s experience in 2020. These stories and topics affected Chapel Hill, Carrboro and the rest of our region.
As the COVID-19 pandemic spread across North Carolina, health officials at UNC worked to gather enough PPE, amp up testing and develop new treatments for the virus. UNC Health joined a national effort to conduct COVID-19 vaccine clinical trials in the midst of a major public health crisis.
As North Carolinians became more aware of the coronavirus in early March, health officials in Orange County tried to prepare for the unknown by gathering enough Personal Protective Equipment (PPE).
Health care systems like UNC Health put out calls for any donations of unopened medical supplies. In April, many of UNC’s professional schools, departments and organizations made substantial efforts to help.
UNC’s Adams School of Dentistry is one example, having donated 99 percent of its PPE after limiting its clinical operations. Dean Scott De Rossi said the school gave approximately 366,000 gloves, 105,000 earloop masks, 1,300 face shields, 600 canisters of disinfectant to the UNC Health system.
“Sending our front line healthcare workers in to manage patients right now without appropriate PPE would be analogous to sending a soldier into battle with a bb gun,” De Rossi said. “It’s not fair to them and potentially dangerous for their patients and co-workers.”
UNC Health first confirmed it was treating a positive COVID-19 case on March 19. Christian Lawson, UNC Medical Center’s Clinical Director of Emergency Services, said the system worked off models that estimated about 30 individual PPE is used to treat one COVID-19 patient.
Lawson said watching the community come together to give during what is a very challenging time for healthcare workers has been very powerful.
“The sheer outpouring of the community really brings back the thought of how great of a country and region we are,” he said in April, “and that there is humanity still out there.”

Gov. Roy Cooper examines a face shield created by Gilero in Pittsboro during a visit there. Gilero is one of the state’s leading producers of PPE. (Photo via the Chatham News + Record.)
Right before confirmation of the system’s first COVID-19 case, UNC Health introduced its first in-house COVID-19 test at its medical center in Chapel Hill on March 16.
With the pandemic in its infantile stages, and with supplies being limited, tests were initially only available for inpatients at UNC Medical Center, UNC REX Hospital and UNC Health affiliate hospitals across North Carolina.
Dr. Melissa Miller is the Director of the Clinical Microbiology and Molecular Microbiology Laboratories for UNC Health Care, which quickly became UNC’s main COVID-19 testing hub. She said it was her mission to roll out an effective test as quickly as possible.
“Quickly creating and validating tests for emerging pathogens has always been part of my passion and commitment, ever since I was a fellow here during SARS,” Miller said. “We have developed a high-quality test, we have the infrastructure to roll it out, and are ready to help the people of our state.”
After months of continuous work, UNC’s Medical Center hit 100,000 completed COVID-19 tests in September. Miller described it as a huge milestone, especially when considering the high demand for testing supplies.
“When we started we could do a maximum of between 120 to 300 a day using our test alone,” Miller said. “Very quickly we built capacity. About two to three weeks after that we had some commercial tests that we brought in-house, which brought us up to the 300 to 500 range. Today we have the capacity to do 2,500 tests per day with the capacity that we built here at the medical center.”

COVID-19 testing at Carrboro High School.
As the rate of positive COVID-19 cases continued to grow from initial cases in the spring into the summer, UNC Health researchers dove headfirst into a nationwide vaccine development effort.
Sites across the country, including one at UNC, took part in testing an mRNA-1273 vaccine developed by Moderna in a large-scale study aiming for 30,000 participants. In August, clinical researchers in the UNC Division of Infectious Diseases asked the community to partake in a large phase 3 clinical trial for a COVID-19 vaccine.
Chapel Hill resident Gracie Howell is just one of the 174 participants recruited at UNC’s site. After losing her corporate job in hospitality due to COVID, Howell became an essential worker at a nearby Wegmans grocery store.
“It became clear to me very early in this situation that a vaccine was going to be the only way out for the world,” Howell said in November.
As clinical trials for a vaccine were underway, new COVID-19 therapeutics were also being developed at UNC.
In October, the U.S. Food and Drug Administration (FDA) approved the antiviral drug Veklury, commonly known as remdesivir, to treat adult and pediatric virus patients. Developed at UNC in partnership with the drug’s manufacturer, remdesivir has been approved or authorized for temporary use as a COVID-19 treatment in approximately 50 countries worldwide.
President Donald Trump received remdesiver along with two other therapeutics while personally being treated for COVID-19 in October.

President Donald Trump stands on the balcony outside of the Blue Room as returns to the White House Monday, Oct. 5, 2020, in Washington, after leaving Walter Reed National Military Medical Center, in Bethesda, Md. Trump announced he tested positive for COVID-19 on Oct. 2. (Photo via AP/Alex Brandon)
FDA Commissioner Dr. Stephen Hahn said the agency was continuously working to bring new COVID-19 treatments to the public as quickly and safely as possible.
“The FDA is committed to expediting the development and availability of COVID-19 treatments during this unprecedented public health emergency,” Hahn said. “Today’s approval is supported by data from multiple clinical trials that the agency has rigorously assessed and represents an important scientific milestone in the COVID-19 pandemic.”
As FDA-approved therapeutics brought hope to frontline workers as they continued to treat patients, the promise of two new vaccines lingered on the horizon.
In November, several COVID-19 vaccine candidates, including the Moderna vaccine stemming from ongoing trials at UNC, worked to gain approval from the FDA.
The FDA said COVID-19 vaccines have to work at least half the time to win approval. Early returns, however, indicated they would be much more effective than that benchmark. In its first release of data, the candidate vaccine made by Pfizer proved 90 percent effective against the virus.
Pfizer’s vaccine was the first to gain approval from the FDA and initial shipments went to the United States in mid-December. It has now been joined by a vaccine from Moderna, which was developed in closer cooperation with scientists from the National Institutes of Health. Moderna said its COVID-19 vaccine is 94.5 percent effective.
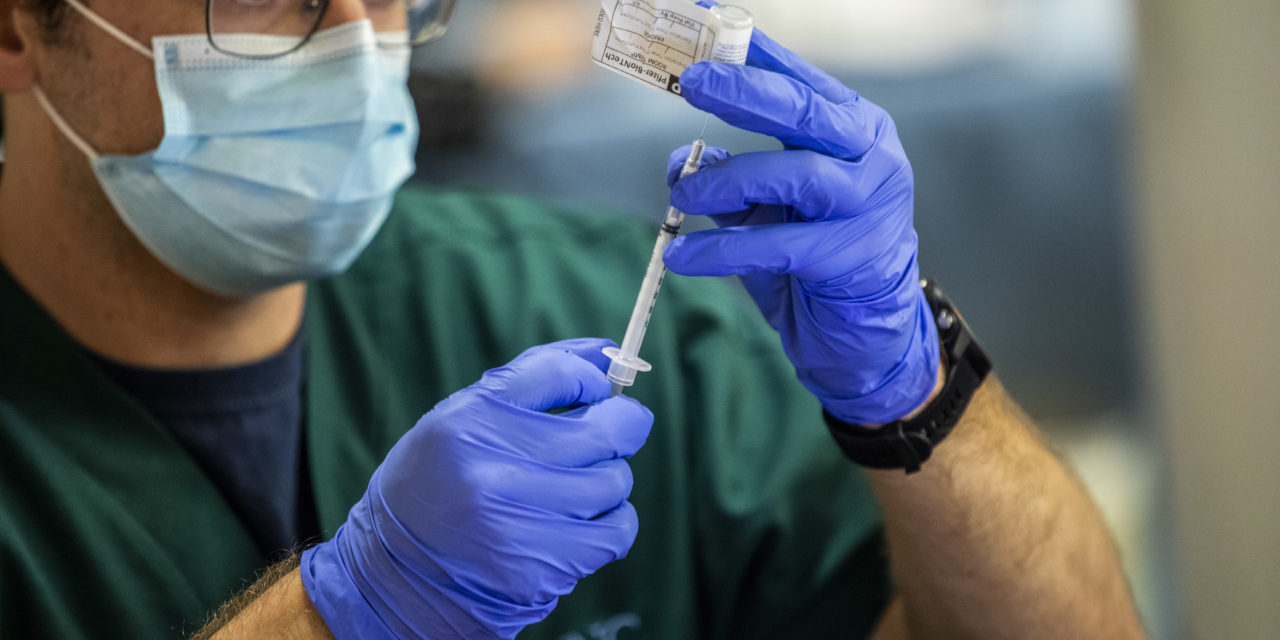
As the first wave of COVID-19 vaccines was distributed across North Carolina, the UNC Medical Center in Chapel Hill was one of the first hospital locations to receive doses on December 15.
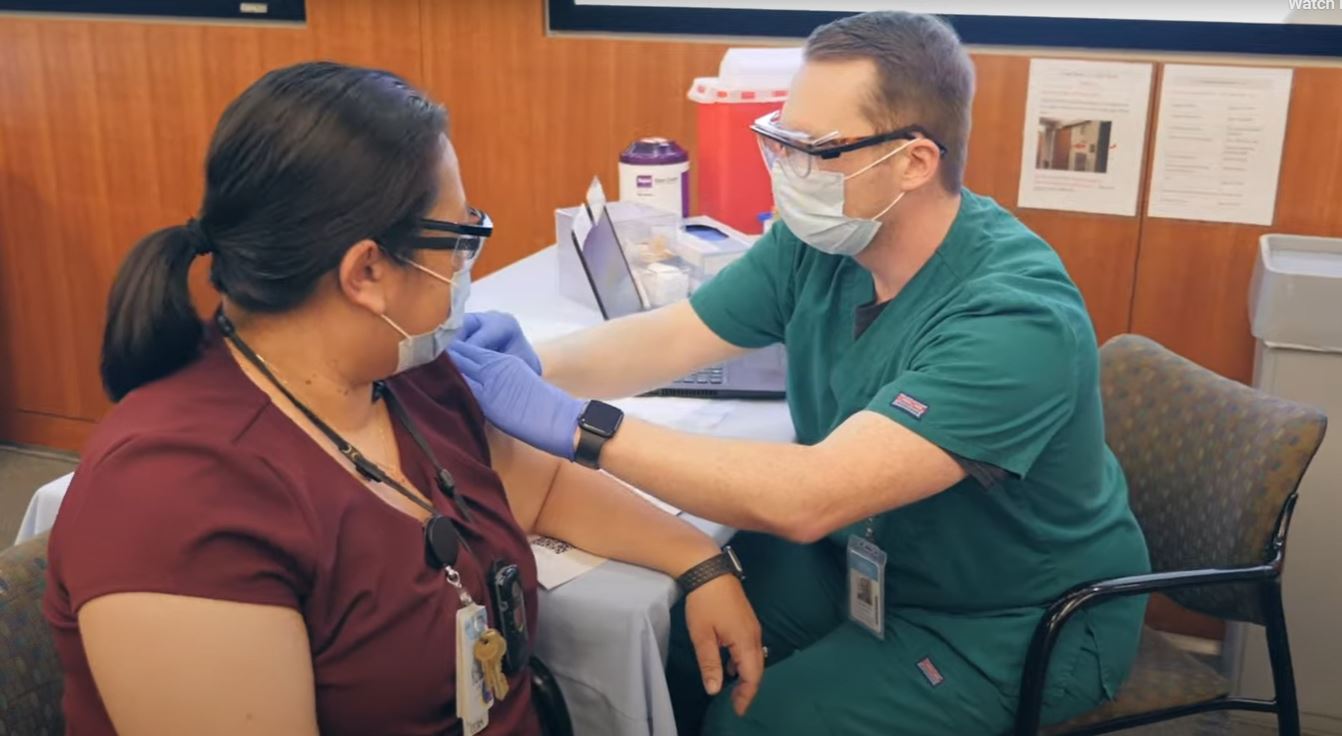
Healthcare workers at the UNC Medical Center in Chapel Hill began receiving COVID-19 vaccines Dec. 15. (Photo via UNC Health)
In coordination with the North Carolina Department of Health and Human Services, hospital staff deemed to be at regular and high risk of exposure to the coronavirus will be the first to receive the emergency vaccine created by Pfizer. Governor Roy Cooper and Secretary of the state health department Dr. Mandy Cohen said earlier this month that 84,800 doses will go to such workers before other health care workers and long-term care residents receive shots.
The decision to vaccinate health care workers initially comes from recommendations from independent state and federal public health advisory committees, which both NCDHHS and Orange County Health are following. Orange County shared its plans for vaccine distribution just days prior to any healthcare workers receiving their first round of the vaccine.
Dr. David Weber, a professor of medicine, pediatrics and epidemiology at UNC’s School of Medicine, said NCDHHS advises people who have had COVID-19 in the past still should be vaccinated, as the vaccine works to protect individuals against future infection.
Weber said, however, it’s a good idea for those individuals to wait three months before receiving the inoculation due to limited vaccine doses. The state is expected to see supply increases anytime from January to June of 2021.
“During that 90 days after having had COVID,” Weber told Chapelboro, “they are relatively protected against a second bout of COVID. There have been just a handful of people who have been reinfected in less than 90 days. So, it’s reasonable to ask them to wait 90 days before they receive the vaccine. But again, they’re not excluded from the vaccine: it is recommended that they get it.”
Lead photo via Travis Long / News & Observer.
Chapelboro.com does not charge subscription fees. You can support local journalism and our mission to serve the community. Contribute today – every single dollar matters.