Written by LAURAN NEERGAARD and MATTHEW PERRONE
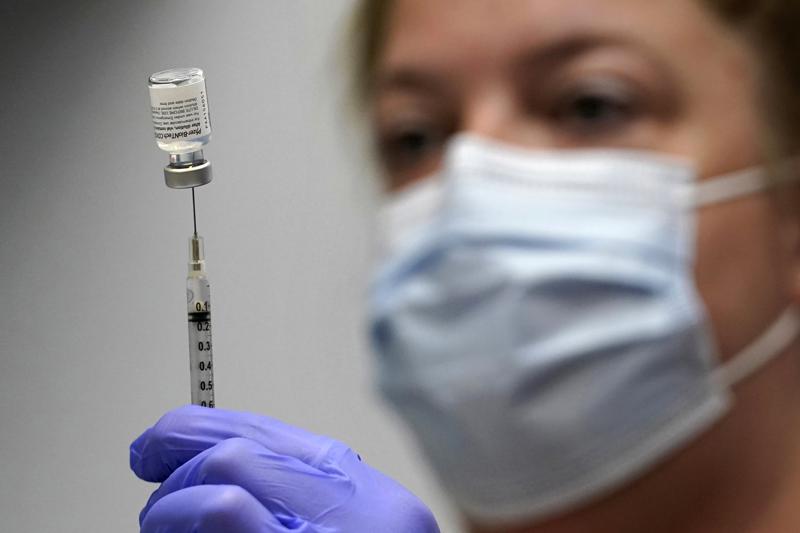
Kid-size doses of Pfizer’s COVID-19 vaccine appear safe and nearly 91% effective at preventing symptomatic infections in 5- to 11-year-olds, according to study details released Friday as the U.S. considers opening vaccinations to that age group.
The shots could begin in early November — with the first children in line fully protected by Christmas — if regulators give the go-ahead.
Details of Pfizer’s study were posted online. The Food and Drug Administration was expected to post its independent review of the company’s safety and effectiveness data later in the day.
Advisers to the FDA will publicly debate the evidence next week. If the agency ultimately authorizes the shots, the Centers for Disease Control and Prevention will make the final decision on who should receive them.
Full-strength Pfizer shots already are authorized for anyone 12 or older, but pediatricians and many parents are anxiously awaiting protection for younger children to stem rising infections from the extra-contagious delta variant and help keep kids in school.
More than 25,000 pediatricians and primary care providers already have signed up to get the shots into little arms.
The Biden administration has purchased enough kid-size doses — in special orange-capped vials to distinguish them from adult vaccine — for the nation’s roughly 28 million 5- to 11-year-olds. If the vaccine is cleared, millions of doses will be promptly shipped around the country, along with kid-size needles.
A Pfizer study tracked 2,268 kids in that age group who got two shots three weeks apart of either a placebo or the low-dose vaccine. Each dose was one-third the amount given to teens and adults.
Researchers calculated the low-dose vaccine was nearly 91% effective, based on 16 COVID-19 cases in youngsters given dummy shots versus three cases among vaccinated children. There were no severe illnesses reported among any of the youngsters, but the vaccinated ones had much milder symptoms than their unvaccinated counterparts.
In addition, young children given the low-dose shots developed coronavirus-fighting antibody levels just as strong as teens and young adults who got regular-strength vaccinations.
That’s important information considering that hospitalizations of mostly unvaccinated children reached record levels last month.
The CDC reported earlier this week that even as the delta mutant surged between June and September, Pfizer vaccinations were 93% effective at preventing hospitalizations among 12- to 18-year-olds.
Pfizer’s study of younger kids found the low-dose shots proved safe, with similar or fewer temporary side effects such as sore arms, fever or achiness that teens experience.
The study isn’t large enough to detect any extremely rare side effects, such as the heart inflammation that occasionally occurs after the second dose, mostly in young men.
While children run a lower risk of severe illness or death than older people, COVID-19 has killed more than 630 Americans 18 and under, according to the CDC. Nearly 6.2 million children have been infected with the coronavirus, more than 1.1 million in the last six weeks as the delta mutant surged, the American Academy of Pediatrics says.
Moderna also is studying its COVID-19 shots in elementary school-age youngsters. Pfizer and Moderna are studying even younger children as well, down to 6-month-olds. Results are expected later in the year.
Photo via AP Photo/Robert F. Bukaty.
Related Stories
‹

US Clears Updated COVID Boosters Targeting Newest VariantsWritten by LAURAN NEERGAARD The U.S. on Wednesday authorized its first update to COVID-19 vaccines, booster doses that target today’s most common omicron strain. Shots could begin within days. The move by the Food and Drug Administration tweaks the recipe of shots made by Pfizer and rival Moderna that already have saved millions of lives. The hope […]
![]()
Marathon US Hearings To Decide Fate of COVID Shots for TotsWritten by LAURAN NEERGAARD Parents anxious to finally vaccinate their youngest children against COVID-19, strap in: A lot is set to happen over the next week. On Wednesday, both Moderna and Pfizer will have to convince what’s essentially a science court — advisers to the Food and Drug Administration — that their shots work well in babies, toddlers and […]

Few North Carolina Children 5-11 Vaccinated in Early RolloutWritten by BRYAN ANDERSON Few North Carolina parents had their children vaccinated in the first days COVID-19 shots were available for kids age 5 to 11, according to the latest data collected by the state Department of Health and Human Services. Dr. Mandy Cohen, the state’s top public health official, said in a Wednesday news […]
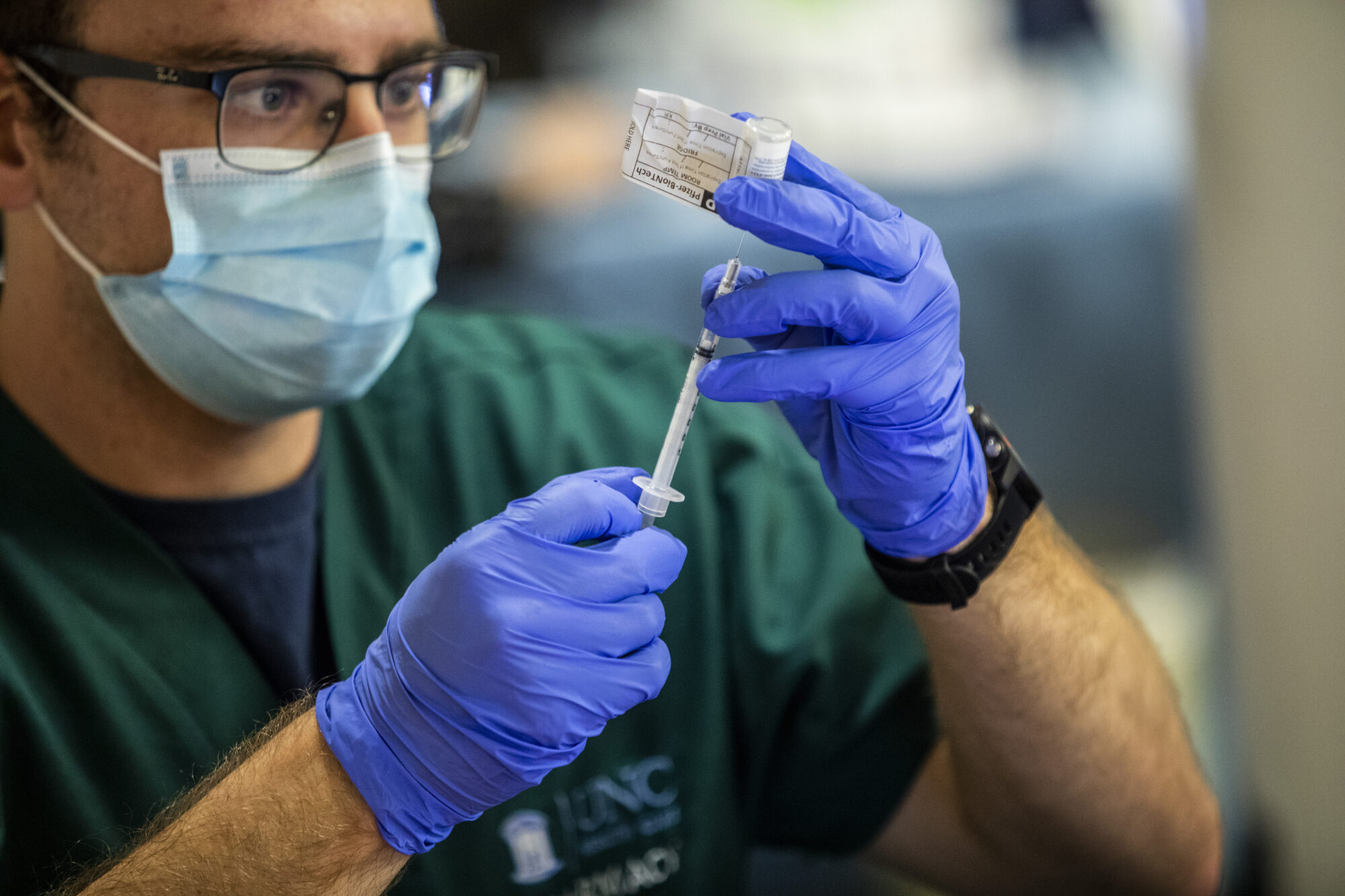
Pfizer Says COVID-19 Vaccine Works in Kids Ages 5 to 11Written by LAURAN NEERGAARD Pfizer said Monday its COVID-19 vaccine works for children ages 5 to 11 and that it will seek U.S. authorization for this age group soon — a key step toward beginning vaccinations for youngsters. The vaccine made by Pfizer and its German partner BioNTech already is available for anyone 12 and […]
![]()
What Does Full Approval of Pfizer’s COVID-19 Vaccine Mean?Written by MATTHEW PERRONE What does full approval of Pfizer’s COVID-19 vaccine mean? It means Pfizer’s shot for people 16 and older has now undergone the same rigorous testing and regulatory review as dozens of other long-established vaccines. COVID-19 vaccines in the U.S. were initially rolled out under the Food and Drug Administration’s emergency use […]
US Regulators Give Full Approval to Pfizer COVID-19 VaccineWritten by LAURAN NEERGAARD and MATTHEW PERRONE The U.S. gave full approval to Pfizer’s COVID-19 vaccine on Monday, a milestone that may help lift public confidence in the shots as the nation battles the most contagious coronavirus mutant yet. The vaccine made by Pfizer and its partner BioNTech now carries the strongest endorsement from the […]

Medically At-Risk North Carolinians Can Get Third COVID ShotWritten by BRYAN ANDERSON North Carolina health officials said Monday that medically vulnerable residents with certain health conditions can get an additional dose of COVID-19 vaccine, though some have already had a third Pfizer or Moderna shot after the U.S. Food and Drug Administration approved it last week. The FDA signed off on the additional dose after […]

Pfizer COVID-19 Shot Expanded to U.S. Children as Young as 12Written by LAURAN NEERGAARD and CANDICE CHOI U.S. regulators on Monday expanded the use of Pfizer’s COVID-19 vaccine to children as young as 12, offering a way to protect the nation’s adolescents before they head back to school in the fall and paving the way for them to return to more normal activities. Shots could begin […]
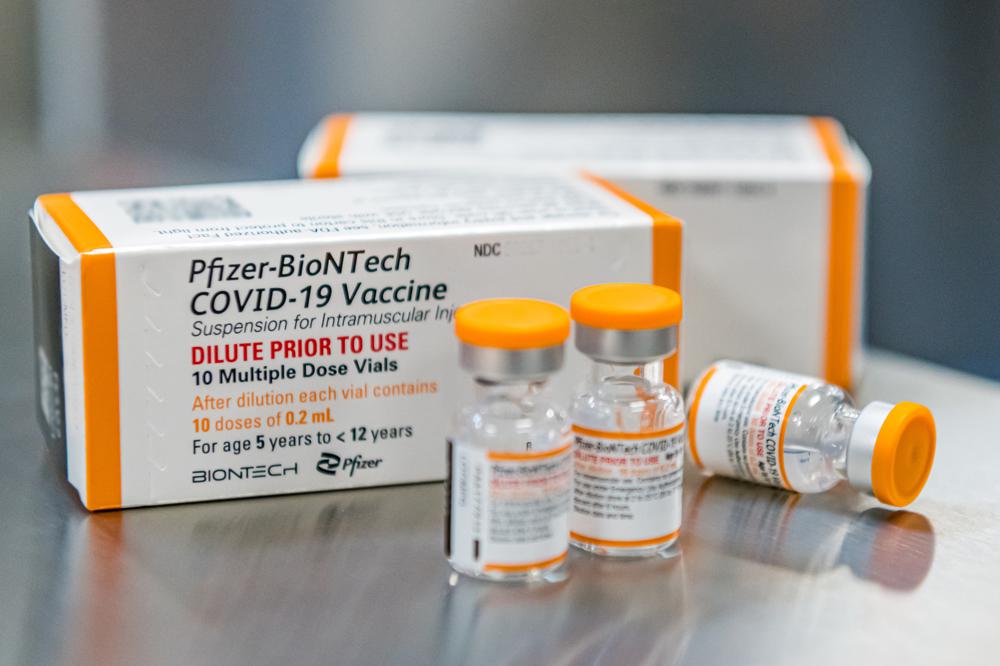
Officials: Millions of COVID-19 Shots Ordered for YoungestMillions of COVID-19 vaccine doses have been ordered for small children in anticipation of possible federal authorization next week, White House officials say.
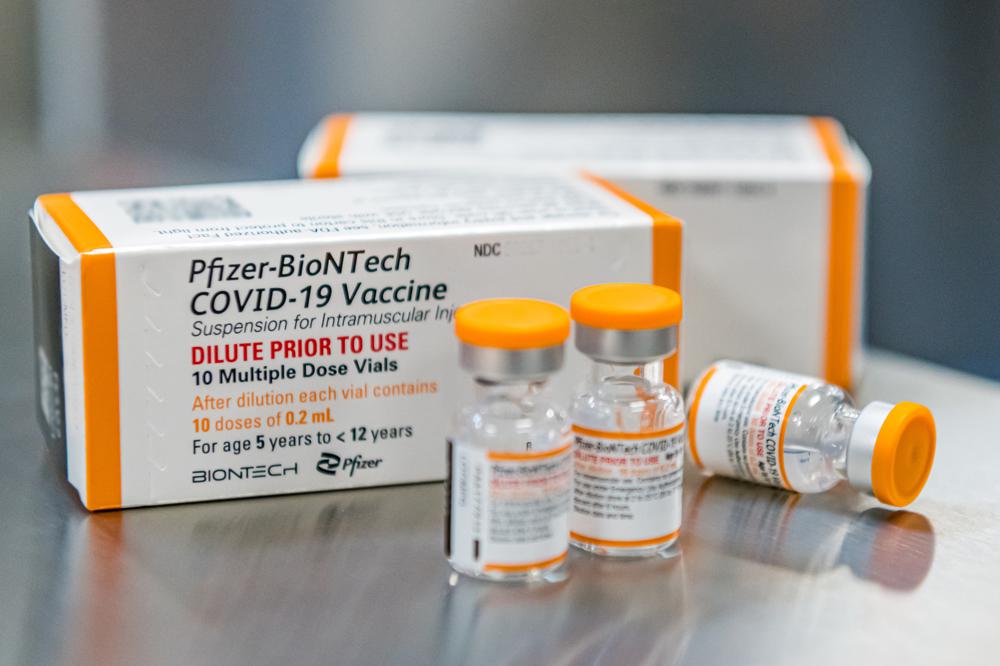
Pfizer Asks FDA To Allow COVID-19 Vaccine for Kids Under 5Written by LAURAN NEERGAARD and MATTHEW PERRONE Pfizer on Tuesday asked the U.S. to authorize extra-low doses of its COVID-19 vaccine for children under 5, potentially opening the way for the very youngest Americans to start receiving shots as early as March. In an extraordinary move, the Food and Drug Administration had urged Pfizer and […]
›








Comments on Chapelboro are moderated according to our Community Guidelines