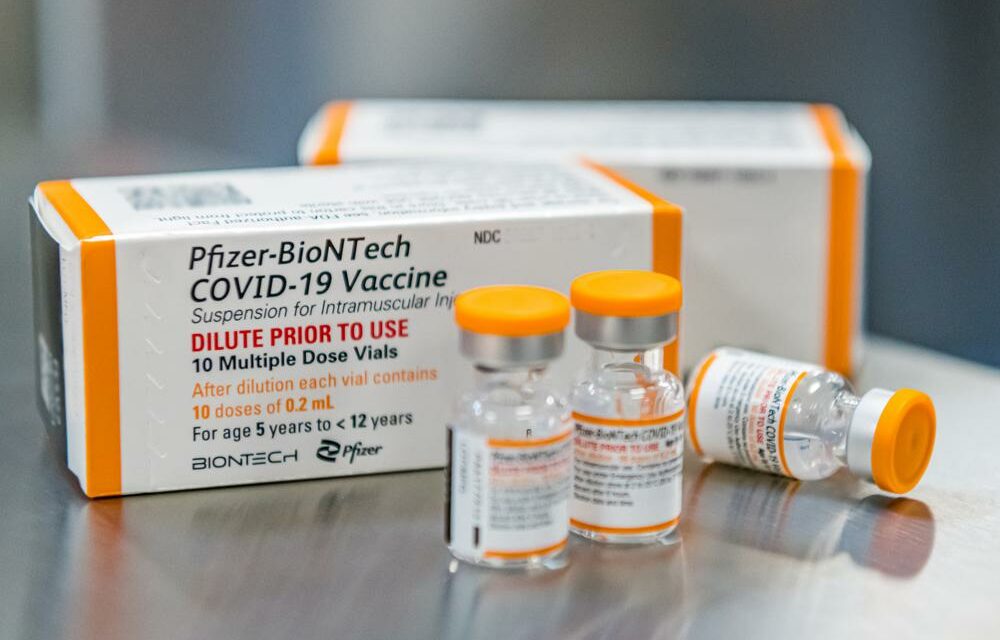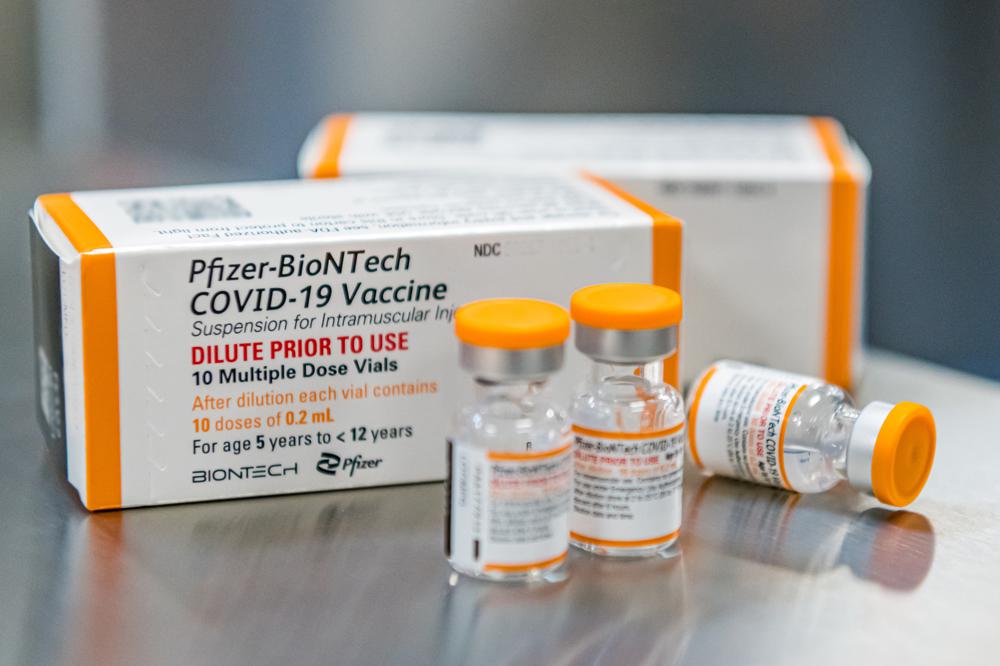Written by MIKE STOBBE
Millions of COVID-19 vaccine doses have been ordered for small children in anticipation of possible federal authorization next week, White House officials say.
The government allowed pharmacies and states to start placing orders last week, with 5 million doses initially available — half of them shots made by Pfizer and the other half the vaccine produced by Moderna, senior administration officials said.
As of this week, about 1.45 million of the 2.5 million available doses of Pfizer have been ordered, and about 850,000 of available Moderna shots have been ordered, officials said. More orders are expected in the coming days.
Young children are the last group of Americans who have not been recommended to get COVID-19 vaccinations. Up to about 20 million U.S. children under 5 would become eligible for vaccination if the government authorizes one or both shots.
It’s not clear how popular the shots will be. A recent survey suggests only 1 in 5 parents of young children would get their kids vaccinated right away.
And public health officials have been disappointed at how many older U.S. children, who have been eligible for shots for months, have yet to be vaccinated: Less than one-third of kids ages 5 to 11 have gotten the two recommended doses, according to government figures.
“As we go down in the age groups, we see lower and lower uptake” of vaccines, said Dr. Lucia Abascal of the California Department of Public Health.
Pfizer has asked FDA to authorize three doses of its COVID-19 vaccine for children ages 6 month to 4 years. Each dose is one-tenth of the amount adults receive.
Moderna has asked FDA to authorize two shots for kids ages 6 months to 5 years, each containing a quarter of the dose given to adults.
The Food and Drug Administration authorizes the use of vaccines, while the Centers for Disease Control and Prevention issues recommendations to doctors and the public about using them.
An FDA advisory committee is scheduled to meet Tuesday and Wednesday to review data from the two companies. Officials say they expect a FDA decision shortly after that meeting.
A CDC advisory committee is scheduled for next Friday and Saturday, with a CDC decision expected soon after.
Vaccinations should begin in earnest as early as June 21, White House COVID-19 coordinator Dr. Ashish Jha told reporters last week.
Photo via Pfizer and Associated Press.
Related Stories
‹
National Expert: Getting Kids Vaccinated Before School is 'Best Thing to Do'Younger children are the age demographic that had the longest waits for COVID-19 vaccines to be approved. So far, their vaccination rates reflect that gap of time, but urgency around getting those initial doses has slowed to a crawl. With a new school year being just around the corner, though, federal health officials are saying […]
How Will Pandemic End? Omicron Clouds Forecasts for EndgameWritten by LAURAN NEERGAARD and CARLA K. JOHNSON Pandemics do eventually end, even if omicron is complicating the question of when this one will. But it won’t be like flipping a light switch: The world will have to learn to coexist with a virus that’s not going away. The ultra-contagious omicron mutant is pushing cases […]
![]()
Vaccines, Masks? Japan Puzzling Over Sudden Virus SuccessWritten by MARI YAMAGUCHI Almost overnight, Japan has become a stunning, and somewhat mysterious, coronavirus success story. Daily new COVID-19 cases have plummeted from a mid-August peak of nearly 6,000 in Tokyo, with caseloads in the densely populated capital now routinely below 100, an 11-month low. The bars are packed, the trains are crowded, and […]

Pfizer COVID-19 Shot Expanded to U.S. Children as Young as 12Written by LAURAN NEERGAARD and CANDICE CHOI U.S. regulators on Monday expanded the use of Pfizer’s COVID-19 vaccine to children as young as 12, offering a way to protect the nation’s adolescents before they head back to school in the fall and paving the way for them to return to more normal activities. Shots could begin […]
5 Things We Know and Still Don’t Know About COVID, 5 Years After It AppearedCOVID-19 is less deadly than it was in the pandemic’s early days. But the virus is evolving, meaning scientists must track it closely.

WHO Downgrades COVID Pandemic, Says It’s No Longer EmergencyWritten by MARIA CHENG and JAMEY KEATEN The World Health Organization said Friday that COVID-19 no longer qualifies as a global emergency, marking a symbolic end to the devastating coronavirus pandemic that triggered once-unthinkable lockdowns, upended economies worldwide and killed at least 7 million people worldwide. WHO first declared COVID-19 to be an emergency more […]

Afraid of Needles? China Rolling Out Oral COVID-19 VaccineWritten by KEN MORITSUGU The Chinese city of Shanghai started administering an inhalable COVID-19 vaccine on Wednesday in what appears to be a world first. The vaccine, a mist that is sucked in through the mouth, is being offered for free as a booster dose for previously vaccinated people, according to an announcement on an […]

US Clears Updated COVID Boosters Targeting Newest VariantsWritten by LAURAN NEERGAARD The U.S. on Wednesday authorized its first update to COVID-19 vaccines, booster doses that target today’s most common omicron strain. Shots could begin within days. The move by the Food and Drug Administration tweaks the recipe of shots made by Pfizer and rival Moderna that already have saved millions of lives. The hope […]

New Coronavirus Mutant Raises Concerns in India and BeyondWritten by LAURA UNGAR and ANIRUDDHA GHOSAL The quickly changing coronavirus has spawned yet another super contagious omicron mutant that’s worrying scientists as it gains ground in India and pops up in numerous other countries, including the United States. Scientists say the variant – called BA.2.75 – may be able to spread rapidly and get […]
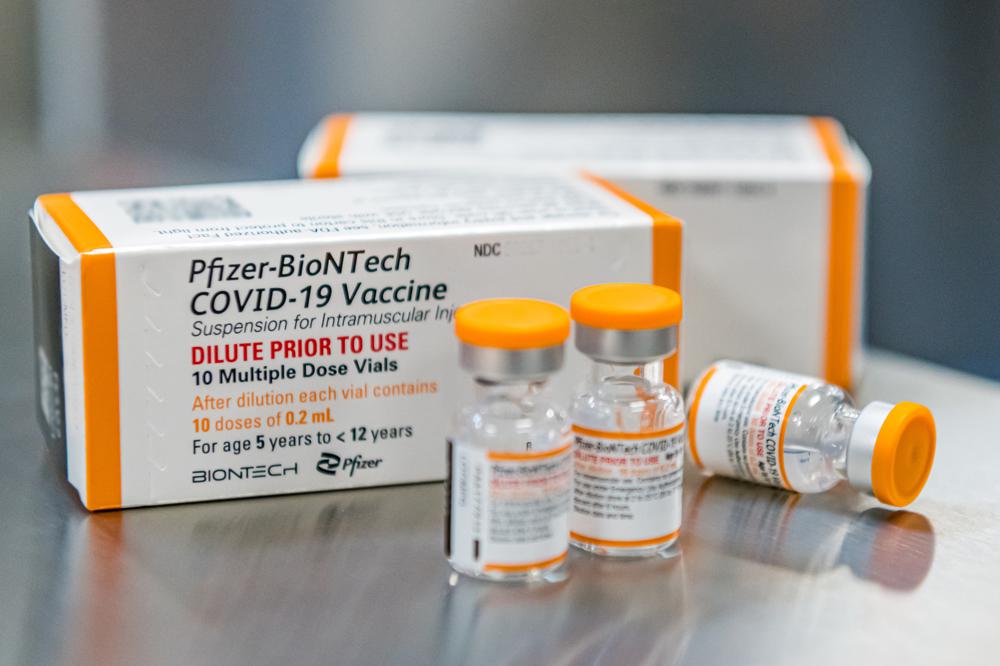
Officials: Millions of COVID-19 Shots Ordered for YoungestMillions of COVID-19 vaccine doses have been ordered for small children in anticipation of possible federal authorization next week, White House officials say.
›