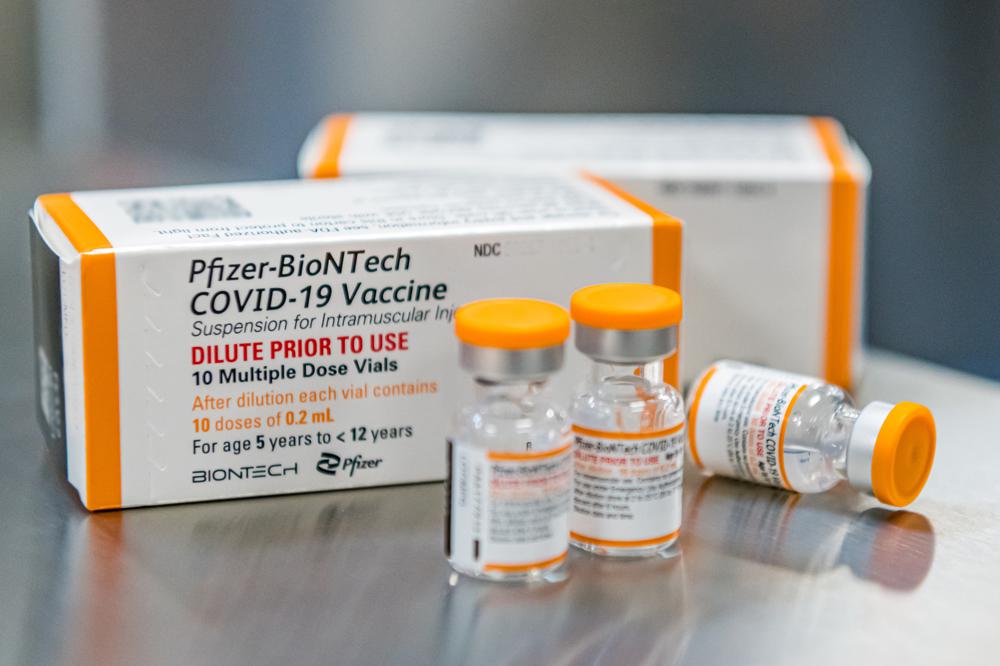Written by LAURAN NEERGAARD and MATTHEW PERRONE
Kid-size doses of Pfizer’s COVID-19 vaccine appear safe and nearly 91% effective at preventing symptomatic infections in 5- to 11-year-olds, according to study details released Friday as the U.S. considers opening vaccinations to that age group.
The shots could begin in early November — with the first children in line fully protected by Christmas — if regulators give the go-ahead.
Details of Pfizer’s study were posted online. The Food and Drug Administration was expected to post its independent review of the company’s safety and effectiveness data later in the day.
Advisers to the FDA will publicly debate the evidence next week. If the agency ultimately authorizes the shots, the Centers for Disease Control and Prevention will make the final decision on who should receive them.
Full-strength Pfizer shots already are authorized for anyone 12 or older, but pediatricians and many parents are anxiously awaiting protection for younger children to stem rising infections from the extra-contagious delta variant and help keep kids in school.
More than 25,000 pediatricians and primary care providers already have signed up to get the shots into little arms.
The Biden administration has purchased enough kid-size doses — in special orange-capped vials to distinguish them from adult vaccine — for the nation’s roughly 28 million 5- to 11-year-olds. If the vaccine is cleared, millions of doses will be promptly shipped around the country, along with kid-size needles.
A Pfizer study tracked 2,268 kids in that age group who got two shots three weeks apart of either a placebo or the low-dose vaccine. Each dose was one-third the amount given to teens and adults.
Researchers calculated the low-dose vaccine was nearly 91% effective, based on 16 COVID-19 cases in youngsters given dummy shots versus three cases among vaccinated children. There were no severe illnesses reported among any of the youngsters, but the vaccinated ones had much milder symptoms than their unvaccinated counterparts.
In addition, young children given the low-dose shots developed coronavirus-fighting antibody levels just as strong as teens and young adults who got regular-strength vaccinations.
That’s important information considering that hospitalizations of mostly unvaccinated children reached record levels last month.
The CDC reported earlier this week that even as the delta mutant surged between June and September, Pfizer vaccinations were 93% effective at preventing hospitalizations among 12- to 18-year-olds.
Pfizer’s study of younger kids found the low-dose shots proved safe, with similar or fewer temporary side effects such as sore arms, fever or achiness that teens experience.
The study isn’t large enough to detect any extremely rare side effects, such as the heart inflammation that occasionally occurs after the second dose, mostly in young men.
While children run a lower risk of severe illness or death than older people, COVID-19 has killed more than 630 Americans 18 and under, according to the CDC. Nearly 6.2 million children have been infected with the coronavirus, more than 1.1 million in the last six weeks as the delta mutant surged, the American Academy of Pediatrics says.
Moderna also is studying its COVID-19 shots in elementary school-age youngsters. Pfizer and Moderna are studying even younger children as well, down to 6-month-olds. Results are expected later in the year.
Photo via AP Photo/Robert F. Bukaty.
Related Stories
‹
National Expert: Getting Kids Vaccinated Before School is 'Best Thing to Do'Younger children are the age demographic that had the longest waits for COVID-19 vaccines to be approved. So far, their vaccination rates reflect that gap of time, but urgency around getting those initial doses has slowed to a crawl. With a new school year being just around the corner, though, federal health officials are saying […]
UNC Health Hits 500,000 Mark of Administered COVID-19 VaccinesWhile COVID-19 vaccinations have drastically slowed in North Carolina, the UNC Health system across the state reached a milestone this week. The health care system shared a release on Friday saying its hospitals and clinics have now administered more than 500,000 coronavirus vaccine doses, reaching the mark in just more than one year of the […]
India Starts Booster Shots for Vulnerable Amid Omicron SurgeWritten by ANIRUDDHA GHOSAL Healthcare and front-line workers along with people above age 60 with health problems lined up Monday at vaccination centers across India to receive a third dose as infections linked to the omicron variant surge. The doses, which India is calling a “precautionary” shot instead of a booster, were given as new […]
![]()
Supreme Court Weighs Vaccine Rules Affecting More Than 80MWritten by MARK SHERMAN and JESSICA GRESKO The Supreme Court began hearing arguments Friday on major Biden administration efforts to bump up the nation’s vaccination rate against COVID-19 at a time of spiking coronavirus cases because of the omicron variant. The justices on the conservative-oriented court were taking up the questions of whether to allow the […]
How Will Pandemic End? Omicron Clouds Forecasts for EndgameWritten by LAURAN NEERGAARD and CARLA K. JOHNSON Pandemics do eventually end, even if omicron is complicating the question of when this one will. But it won’t be like flipping a light switch: The world will have to learn to coexist with a virus that’s not going away. The ultra-contagious omicron mutant is pushing cases […]

Few North Carolina Children 5-11 Vaccinated in Early RolloutWritten by BRYAN ANDERSON Few North Carolina parents had their children vaccinated in the first days COVID-19 shots were available for kids age 5 to 11, according to the latest data collected by the state Department of Health and Human Services. Dr. Mandy Cohen, the state’s top public health official, said in a Wednesday news […]
![]()
Hillsborough: Kid Vaccines, NDO Updates and A Clean-UpHillsborough Mayor Jenn Weaver joins 97.9 The Hill's Brighton McConnell on Tuesday, November 9. She discusses her experience of children 5-11 years-old being now eligible for COVID-19 vaccines. Plus, from the local government: updates from the latest Board of Commissioners meeting and early discussions of when they might return to in-person again.
US Mandates Vaccines or Tests for Big Companies by Jan. 4Written by DAVID KOENIG Tens of millions of Americans who work at companies with 100 or more employees will need to be fully vaccinated against COVID-19 by Jan. 4 or get tested for the virus weekly under government rules issued Thursday. The new requirements, which were first previewed by President Joe Biden in September, will […]
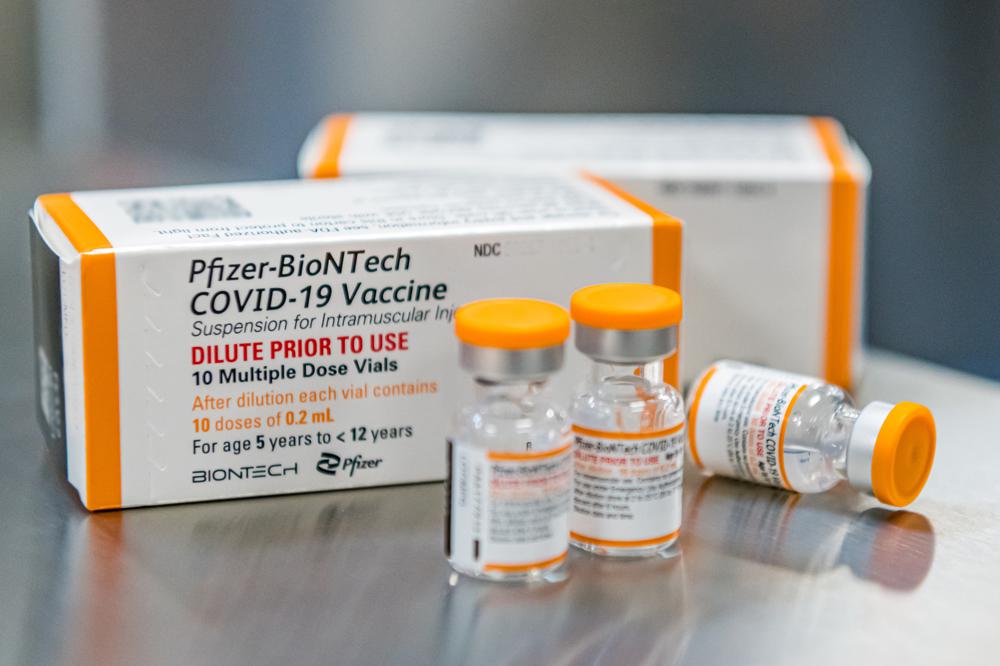
US Gives Final Clearance to COVID-19 Shots for Kids 5 to 11U.S. health officials on Tuesday gave the final signoff to Pfizer’s kid-size COVID-19 shot, a milestone that opens a major expansion of the nation’s vaccination campaign to children as young as 5. The Food and Drug Administration already authorized the shots for children ages 5 to 11 — doses just a third of the amount […]
Pfizer Says COVID-19 Vaccine More Than 90% Effective in KidsWritten by LAURAN NEERGAARD and MATTHEW PERRONE Kid-size doses of Pfizer’s COVID-19 vaccine appear safe and nearly 91% effective at preventing symptomatic infections in 5- to 11-year-olds, according to study details released Friday as the U.S. considers opening vaccinations to that age group. The shots could begin in early November — with the first children […]
›