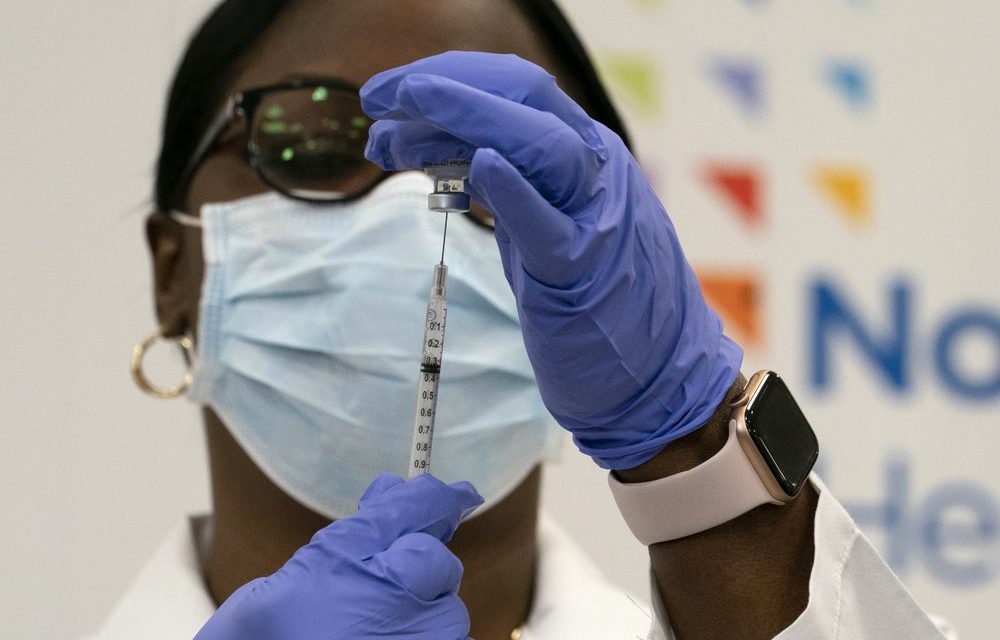
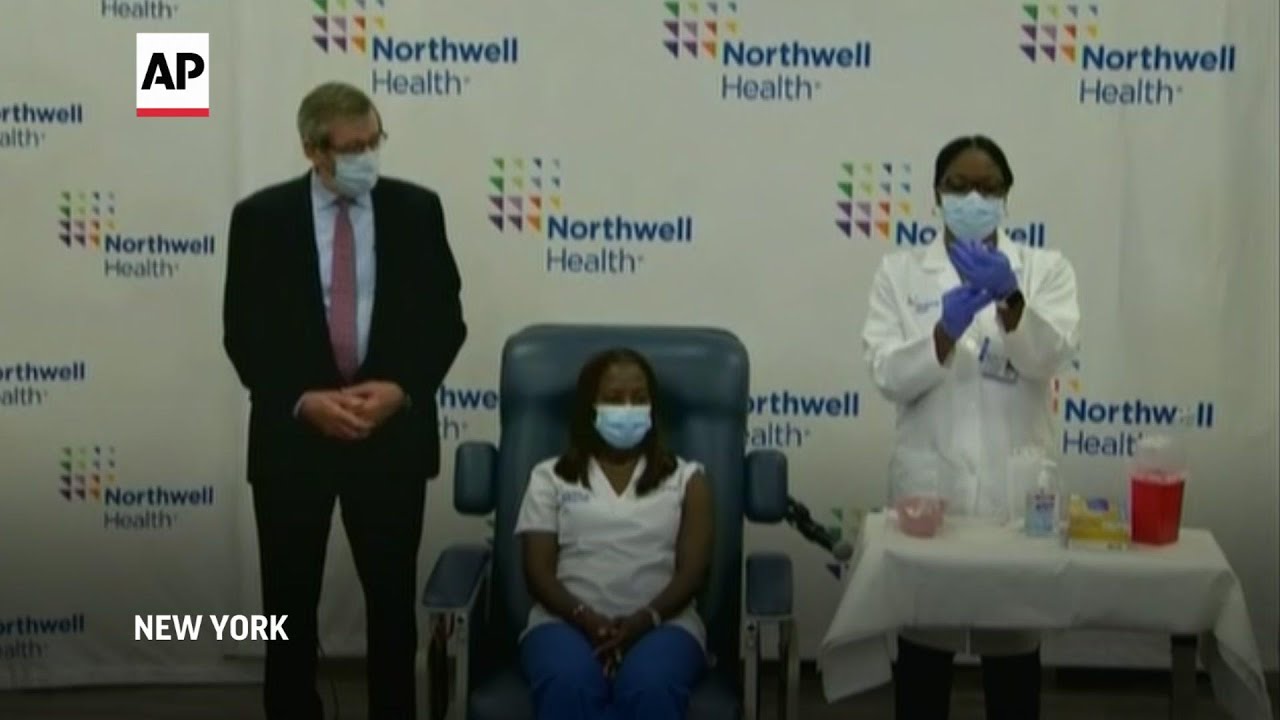
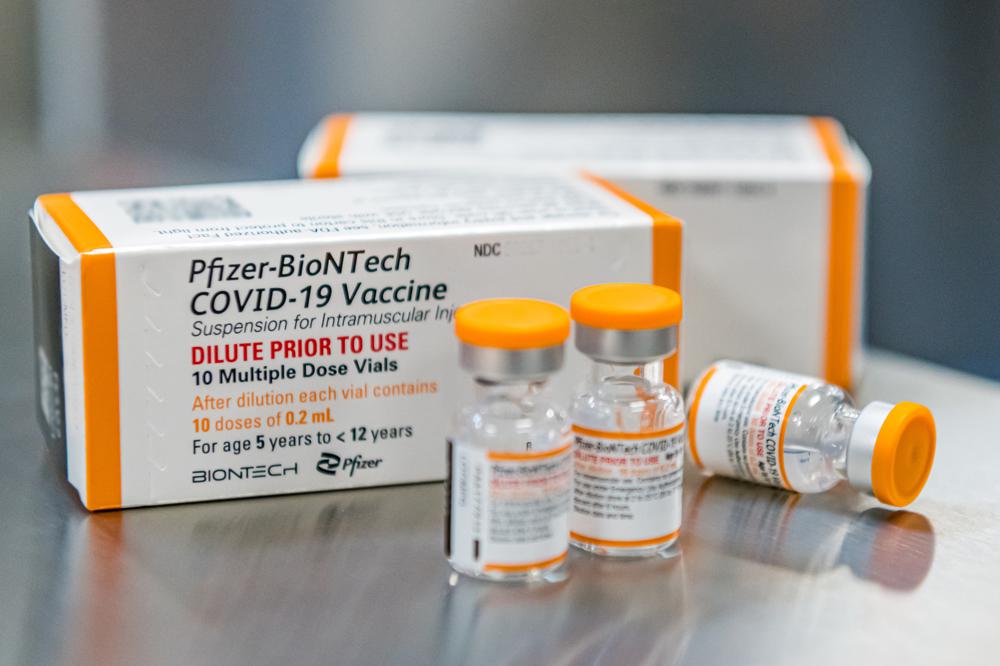The largest vaccination campaign in U.S. history got underway Monday as health workers in select hospitals rolled up their sleeves for shots to protect them from COVID-19 and start beating back the pandemic — a day of optimism even as the nation’s death toll neared 300,000.
“I feel hopeful today. Relieved,” said critical case nurse Sandra Lindsay after getting a shot in the arm at Long Island Jewish Medical Center in New York.
Shipments of precious frozen vials of vaccine made by Pfizer Inc. and its German partner BioNTech began arriving at hospitals around the country Monday.
“This is the light at the end of the tunnel. But it’s a long tunnel,” New York Gov. Andrew Cuomo said as he watched Lindsay’s vaccination via video.
Several other countries also have OK’d the vaccine, including the U.K., which started vaccinating last week.
For health care workers who, along with nursing home residents, will be first in line for vaccination, hope is tempered by grief and the sheer exhaustion of months spent battling a coronavirus that still is surging in the U.S. and around the world.
“This is mile 24 of a marathon. People are fatigued. But we also recognize that this end is in sight,” said Dr. Chris Dale of Swedish Health Services in Seattle.
Packed in dry ice to stay at ultra-frozen temperatures,
the first of nearly 3 million doses being shipped in staggered batches this week made their way by truck and by plane around the country Sunday from Pfizer’s Kalamazoo, Michigan, factory. Once they arrive at distribution centers, each state directs where the doses go next.
Some hospitals across the country spent the weekend tracking their packages, refreshing FedEx and UPS websites for clues.
More of the Pfizer-BioNTech vaccine will arrive each week. And later this week, the FDA will decide whether to green light the world’s second rigorously studied COVID-19 vaccine, made by Moderna Inc.
Now the hurdle is to rapidly get vaccine into the arms of millions, not just doctors and nurses but other at-risk health workers such as janitors and food handlers — and then deliver a second dose three weeks later.
“We’re also in the middle of a surge, and it’s the holidays, and our health care workers have been working at an extraordinary pace,” said Sue Mashni, chief pharmacy officer at Mount Sinai Health System in New York City.
Plus, the shots can cause temporary fever, fatigue and aches as they rev up people’s immune systems, forcing hospitals to stagger employee vaccinations.
A wary public will be watching closely to see whether health workers embrace vaccination. Just half of Americans say they want to get vaccinated, while about a quarter don’t and the rest are unsure, according to a recent poll by The Associated Press-NORC Center for Public Health Research.
The FDA, considered the world’s most strict medical regulator, said the Pfizer-BioNTech vaccine appears safe and strongly protective — and laid out the data behind it in a daylong public meeting last week for scientists and consumers alike to see.
“Please people, when you look back in a year and you say to yourself, ‘Did I do the right thing?’ I hope you’ll be able to say, ‘Yes, because I looked at the evidence,’” Dr. Francis Collins, director of the National Institutes of Health, said Sunday on NBC’s Meet the Press. “People are dying right now. How could you possibly say, ‘Let’s wait and see.’”
Still, emergency use means the vaccine was cleared for widespread use before a final study in nearly 44,000 people is complete — and that research is continuing to try to answer additional questions. While effective against COVID-19 illness, it’s not yet clear if vaccination will stop the symptomless spread that accounts for half of all cases.
The shots still must be studied in children, and during pregnancy. But the American College of Obstetricians and Gynecologists said late Sunday that vaccination should not be withheld from pregnant women who otherwise would qualify.
While the vaccine was determined to be safe, regulators in the U.K. are investigating several severe allergic reactions. The FDA’s instructions tell providers not to give it to those with a known history of severe allergic reactions to any of its ingredients.
Photo via AP Photo/Mark Lennihan.
Related Stories
‹

U.S. Allows Emergency COVID-19 Vaccine in Bid to End PandemicThe U.S. gave the final go-ahead Friday to the nation’s first COVID-19 vaccine, marking what could be the beginning of the end of an outbreak that has killed nearly 300,000 Americans. Shots for health workers and nursing home residents are expected to begin in the coming days after the Food and Drug Administration authorized an […]

Moderna Sues Pfizer Over Patents Behind COVID-19 VaccineWritten by TOM MURPHY COVID-19 vaccine maker Moderna is suing Pfizer and the German drugmaker BioNTech, accusing its main competitors of copying Moderna’s technology in order to make their own vaccine. Moderna said Friday that Pfizer and BioNTech’s vaccine Comirnaty infringes on patents Moderna filed several years ago protecting the technology behind its preventive shot, […]
![]()
U.S. Parents Excited Over Prospect of Virus Shots for ChildrenWritten by HEATHER HOLLINGSWORTH and TODD RICHMOND After more than a year of fretting over her 13-year son with a rare liver disease, Heather Ousley broke into tears when she learned that he and millions of other youngsters could soon be eligible for the COVID-19 vaccine. “This day is the best day in the history of […]

U.S. Boosting Vaccine Deliveries Amid Complaints of ShortagesAnswering growing frustration over vaccine shortages, President Joe Biden announced that the U.S. is ramping up deliveries to hard-pressed states over the next three weeks and expects to provide enough doses to vaccinate 300 million Americans by the end of the summer or early fall. Biden, calling the push a “wartime effort,” said Tuesday the […]

U.S. Close on Deal With Pfizer for Millions More Vaccine DosesThe U.S. government is close to a deal to acquire tens of millions of additional doses of Pfizer’s vaccine in exchange for helping the pharmaceutical giant gain better access to manufacturing supplies. A person with knowledge of the negotiations told The Associated Press on Tuesday that the deal is under discussion and could be finalized […]

U.S. Panel Endorses Widespread Use of Pfizer COVID-19 VaccineA U.S. government advisory panel endorsed widespread use of Pfizer’s coronavirus vaccine Thursday, putting the country just one step away from launching an epic vaccination campaign against the outbreak that has killed close to 300,000 Americans. Shots could begin within days, depending on how quickly the Food and Drug Administration signs off, as expected, on […]
Shots for Tots: NC Begins COVID-19 Vaccines for Young ChildrenThe Centers for Disease Control recently approved both Moderna and Pfizer vaccines for children as young as six months. Locally, Orange County vaccines for the young age group are available starting Monday and by appointment only at the Southern Human Services Center in Chapel Hill.
Pfizer COVID-19 Shot Appears Effective For Kids Under 5Written by MATTHEW PERRONE and MIKE STOBBE Federal health officials said Sunday that kid-sized doses of Pfizer’s COVID-19 vaccines appear to be safe and effective for kids under 5, a key step toward a long-awaited decision to begin vaccinating the youngest American children. The Food and Drug Administration posted its analysis of the Pfizer shot ahead […]
Officials: Millions of COVID-19 Shots Ordered for YoungestMillions of COVID-19 vaccine doses have been ordered for small children in anticipation of possible federal authorization next week, White House officials say.
![]()
Moderna Seeks FDA Authorization for 4th Dose of COVID ShotWritten by ZEKE MILLER Drugmaker Moderna asked the Food and Drug Administration on Thursday to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults. The request is broader than rival pharmaceutical company Pfizer’s request earlier this week for the regulator to approve a booster shot for all seniors. In […]
›