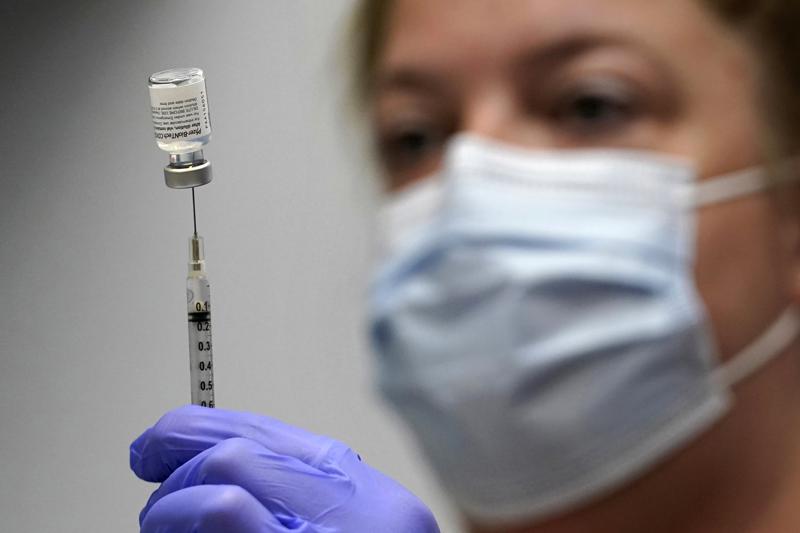
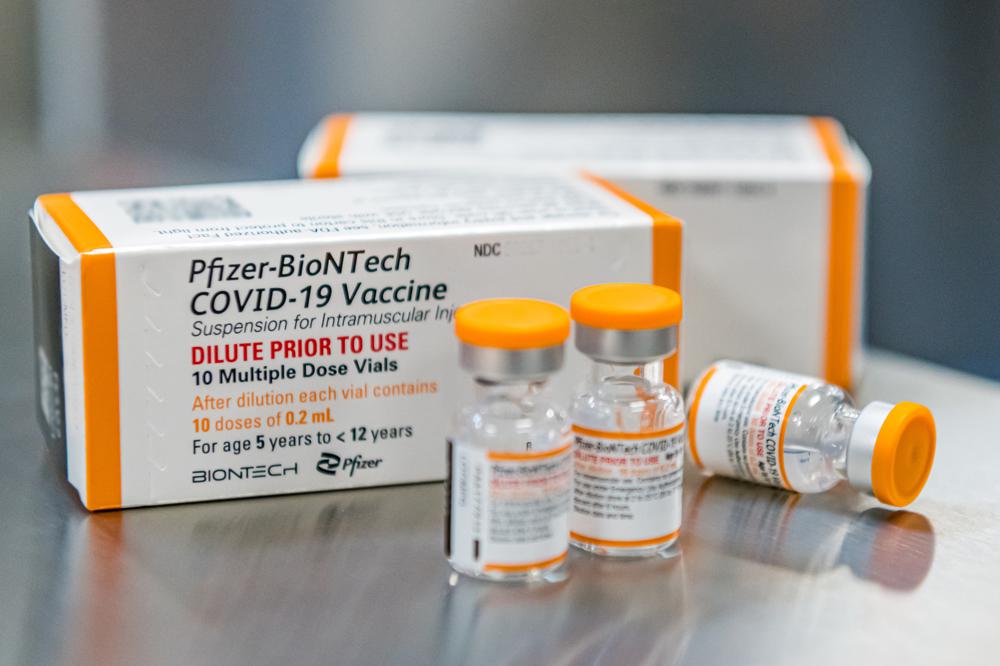Written by LAURAN NEERGAARD
Pfizer asked the U.S. government Thursday to allow use of its COVID-19 vaccine in children ages 5 to 11 — and if regulators agree, shots could begin within a matter of weeks.
Many parents and pediatricians are clamoring for protection for children younger than 12, today’s age cutoff for the vaccine made by Pfizer and its German partner BioNTech. Not only can youngsters sometimes get seriously ill, but keeping them in school can be a challenge with the coronavirus still raging in poorly vaccinated communities.
Pfizer announced in a tweet that it had formally filed its application with the Food and Drug Administration.
Now the FDA will have to decide if there’s enough evidence that the shots are safe and will work for younger children like they do for teens and adults. An independent expert panel will publicly debate the evidence on Oct. 26.
One big change: Pfizer says its research shows the younger kids should get a third of the dose now given to everyone else. After their second dose, the 5- to 11-year-olds developed virus-fighting antibody levels just as strong as teens and young adults get from regular-strength shots.
While kids are at lower risk of severe illness or death than older people, COVID-19 does sometimes kill children and cases in youngsters have skyrocketed as the extra-contagious delta variant has swept through the country
“It makes me very happy that I am helping other kids get the vaccine,” said Sebastian Prybol, 8, of Raleigh, North Carolina. He is enrolled in Pfizer’s study at Duke University and doesn’t yet know if he received the vaccine or dummy shots.
“We do want to make sure that it is absolutely safe for them,” said Sebastian’s mother, Britni Prybol. But she said she will be “overjoyed” if the FDA clears the vaccine.
Pfizer studied the lower dose in 2,268 volunteers ages 5 to 11, and has said there were no serious side effects. The study isn’t large enough to detect any extremely rare side effects, such as the heart inflammation that sometimes occurs after the second dose of the regular-strength vaccine, mostly in young men.
If the FDA authorizes emergency use of the kid-sized doses, there’s another hurdle before vaccinations in this age group can begin. Advisers to the Centers for Disease Control and Prevention will decide whether to recommend the shots for youngsters, and the CDC will make a final decision.
To avoid dosing mix-ups, Pfizer is planning to ship vials specially marked for pediatric use containing the lower dose.
Photo via AP Photo/Robert F. Bukaty.
Related Stories
‹
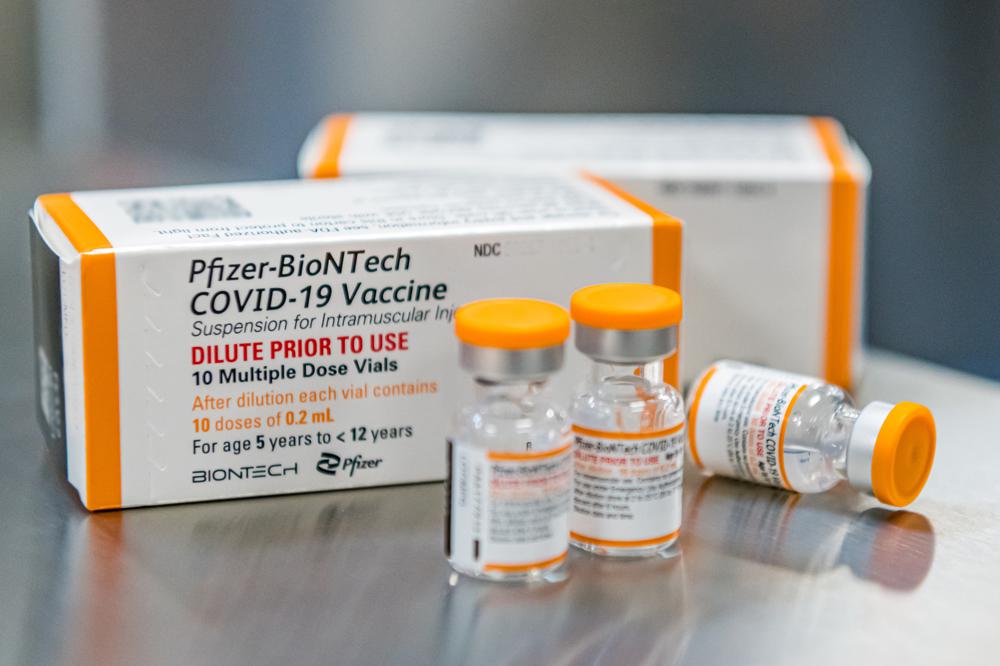
US Gives Final Clearance to COVID-19 Shots for Kids 5 to 11U.S. health officials on Tuesday gave the final signoff to Pfizer’s kid-size COVID-19 shot, a milestone that opens a major expansion of the nation’s vaccination campaign to children as young as 5. The Food and Drug Administration already authorized the shots for children ages 5 to 11 — doses just a third of the amount […]

US Clears Updated COVID Boosters Targeting Newest VariantsWritten by LAURAN NEERGAARD The U.S. on Wednesday authorized its first update to COVID-19 vaccines, booster doses that target today’s most common omicron strain. Shots could begin within days. The move by the Food and Drug Administration tweaks the recipe of shots made by Pfizer and rival Moderna that already have saved millions of lives. The hope […]
![]()
Moderna Seeks FDA Authorization for 4th Dose of COVID ShotWritten by ZEKE MILLER Drugmaker Moderna asked the Food and Drug Administration on Thursday to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults. The request is broader than rival pharmaceutical company Pfizer’s request earlier this week for the regulator to approve a booster shot for all seniors. In […]
US Expands COVID Boosters to All Adults, Final Hurdle AheadWritten by LAURAN NEERGAARD and MATTHEW PERRONE U.S. regulators on Friday moved to open up COVID-19 booster shots to all adults, expanding the government’s campaign to shore up protection and get ahead of rising coronavirus cases that may worsen with the holidays. Pfizer and Moderna announced the Food and Drug Administration’s decision after at least […]
US Regulators Give Full Approval to Pfizer COVID-19 VaccineWritten by LAURAN NEERGAARD and MATTHEW PERRONE The U.S. gave full approval to Pfizer’s COVID-19 vaccine on Monday, a milestone that may help lift public confidence in the shots as the nation battles the most contagious coronavirus mutant yet. The vaccine made by Pfizer and its partner BioNTech now carries the strongest endorsement from the […]
Extra COVID Vaccine OK’d for Those With Weak Immune SystemsWritten by LAURAN NEERGAARD and MATTHEW PERRONE U.S. regulators say transplant recipients and others with severely weakened immune systems can get an extra dose of the Pfizer or Moderna COVID-19 vaccines to better protect them as the delta variant continues to surge. The late-night announcement Thursday by the Food and Drug Administration applies to several […]
![]()
U.S. Parents Excited Over Prospect of Virus Shots for ChildrenWritten by HEATHER HOLLINGSWORTH and TODD RICHMOND After more than a year of fretting over her 13-year son with a rare liver disease, Heather Ousley broke into tears when she learned that he and millions of other youngsters could soon be eligible for the COVID-19 vaccine. “This day is the best day in the history of […]

U.S. Panel Endorses Widespread Use of Pfizer COVID-19 VaccineA U.S. government advisory panel endorsed widespread use of Pfizer’s coronavirus vaccine Thursday, putting the country just one step away from launching an epic vaccination campaign against the outbreak that has killed close to 300,000 Americans. Shots could begin within days, depending on how quickly the Food and Drug Administration signs off, as expected, on […]
Here’s Where Jobs and Programs Are Being Cut at the Nation’s Top Health AgenciesThousands of people were laid off Tuesday at the United States' top health agencies. Here are what positions and efforts were cut.
Moderna Sues Pfizer Over Patents Behind COVID-19 VaccineWritten by TOM MURPHY COVID-19 vaccine maker Moderna is suing Pfizer and the German drugmaker BioNTech, accusing its main competitors of copying Moderna’s technology in order to make their own vaccine. Moderna said Friday that Pfizer and BioNTech’s vaccine Comirnaty infringes on patents Moderna filed several years ago protecting the technology behind its preventive shot, […]
›