Written by TOM MURPHY
The U.S. government will pay drugmaker Pfizer $5.29 billion for 10 million treatment courses of its potential COVID-19 treatment if regulators authorize it, the nation’s largest purchase agreement yet for a coronavirus therapy.
Pfizer asked the Food and Drug Administration on Tuesday to authorize emergency use of the experimental pill, which has been shown to significantly cut the rate of hospitalizations and deaths among people with coronavirus infections.
The FDA is already reviewing a competing pill from Merck and will hold a public meeting on it later this month.
The price for Pfizer’s potential treatment amounts to about $529 per course. The U.S. has already agreed to pay roughly $700 per course of Merck’s drug for about 3.1 million treatments.
Pfizer said Thursday the price being paid by the U.S. government reflects the high number of treatment courses purchased through 2022.
President Joe Biden said in a statement that his administration is taking steps to ensure that the treatments “will be easily accessible and free.”
“This treatment could prove to be another critical tool in our arsenal that will accelerate our path out of the pandemic,” Biden said, adding that vaccines protecting against the virus remain the strongest tool.
Pfizer has started rolling submissions for approval in several other countries and there are advanced purchase agreements with other governments as well.
On Tuesday, Pfizer signed a deal a with U.N.-backed group to allow generic drugmakers to produce low-cost versions of the pill for certain countries. Merck has a similar deal for its pill, which was authorized in Britain earlier this month.
Pfizer reported earlier this month that its pill cut hospitalizations and deaths by 89% among high-risk adults who had early symptoms of COVID-19.
The company studied its pill in people who were unvaccinated and faced the worst risks from the virus due to age or health problems, such as obesity.
Pfizer wants the drug available for adults who have mild-to-moderate COVID-19 infections and are at risk of becoming seriously ill. That’s similar to how other drugs are currently used to treat the disease.
But all FDA-authorized COVID-19 treatments require an IV or injection given by a health professional at a hospital or clinic.
Pfizer’s potential treatment is taken twice a day for five days, in combination with a second antiviral pill that boosts its effect.
Pfizer has already booked more than $24 billion in global revenue so far this year from Comirnaty, its COVID-19 vaccine, which has quickly become the drugmaker’s top-selling product.
Shares of New York-based Pfizer Inc. climbed less than 2% at the opening bell Thursday. They hit an all-time high of $51.86 this summer, topping a previous mark that had stood for 22 years.
Pfizer and Merck are seeking approval for their treatments as COVID-19 cases start to rebound again in the U.S.
The seven-day rolling average for daily new cases approached 87,000 on Wednesday, according to data from Johns Hopkins University. That’s up from around 68,000 late last month.
Photo via Pfizer and the Associated Press.
Related Stories
‹
![]()
FDA: Merck COVID Pill Effective, Experts Will Review SafetyWritten by MATTHEW PERRONE Federal health regulators say an experimental COVID-19 pill from Merck is effective against the virus, but they will seek input from outside experts on risks of birth defects and other potential problems in pregnant women. The Food and Drug Administration posted its analysis of the pill ahead of a public meeting […]
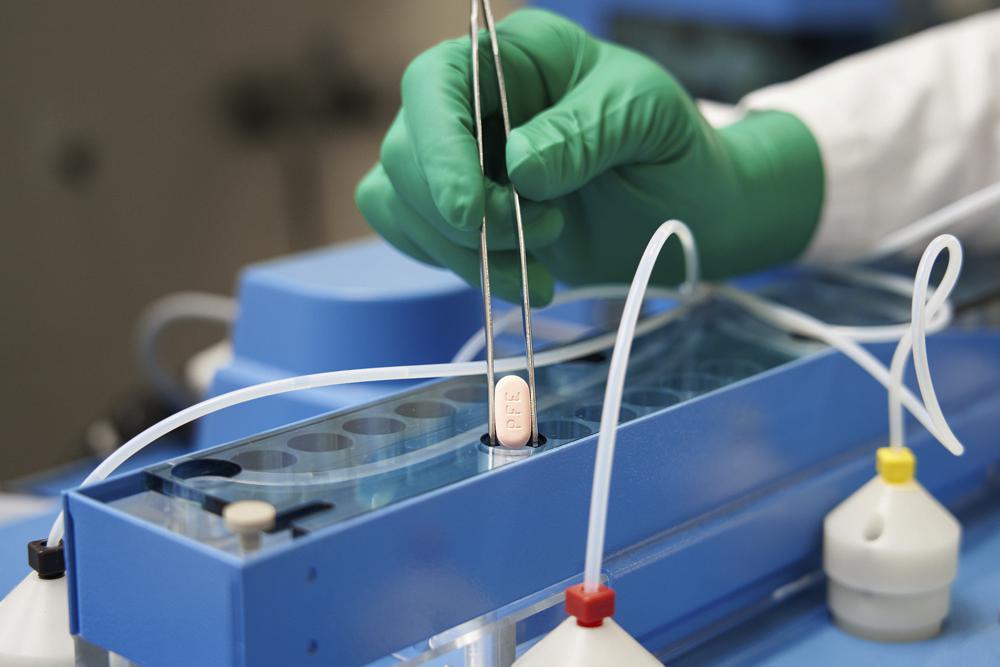
COVID-19 Pill Rollout Stymied by Shortages as Omicron RagesWritten by MATTHEW PERRONE Two brand-new COVID-19 pills that were supposed to be an important weapon against the pandemic in the U.S. are in short supply and have played little role in the fight against the omicron wave of infections. The problem is that production is not yet at full strength and that the pill […]

Pfizer Agrees To Let Other Companies Make Its COVID-19 PillWritten by MARIA CHENG Drugmaker Pfizer Inc. has signed a deal with a U.N.-backed group to allow other manufacturers to make its experimental COVID-19 pill, a move that could make the treatment available to more than half of the world’s population. In a statement issued Tuesday, Pfizer said it would grant a license for the […]
![]()
Pfizer Says COVID-19 Pill Cut Hospital, Death Risk by 90%Written by MATTHEW PERRONE Pfizer Inc. said Friday that its experimental antiviral pill for COVID-19 cut rates of hospitalization and death by nearly 90% in high-risk adults, as the drugmaker joins the race to bring the first easy-to-use medication against the coronavirus to the U.S. market. Currently all COVID-19 treatments used in the U.S. require […]
![]()
UK Authorizes Merck Antiviral Pill, 1st Shown To Treat COVIDWritten by MATTHEW PERRONE and MARIA CHENG Britain granted conditional authorization on Thursday to the first pill shown to successfully treat COVID-19 so far. It also is the first country to OK the treatment from drugmaker Merck, although it wasn’t immediately clear how quickly the pill would be available. The pill was licensed for adults […]
![]()
Merck Asks US FDA To Authorize Promising Anti-COVID PillWritten by MATTHEW PERRONE Drugmaker Merck asked U.S. regulators Monday to authorize its pill against COVID-19 in what would add an entirely new and easy-to-use weapon to the world’s arsenal against the pandemic. If cleared by the Food and Drug Administration — a decision that could come in a matter of weeks — it would […]

Extra COVID Vaccine OK’d for Those With Weak Immune SystemsWritten by LAURAN NEERGAARD and MATTHEW PERRONE U.S. regulators say transplant recipients and others with severely weakened immune systems can get an extra dose of the Pfizer or Moderna COVID-19 vaccines to better protect them as the delta variant continues to surge. The late-night announcement Thursday by the Food and Drug Administration applies to several […]

U.S. Close on Deal With Pfizer for Millions More Vaccine DosesThe U.S. government is close to a deal to acquire tens of millions of additional doses of Pfizer’s vaccine in exchange for helping the pharmaceutical giant gain better access to manufacturing supplies. A person with knowledge of the negotiations told The Associated Press on Tuesday that the deal is under discussion and could be finalized […]

U.S. Panel Endorses Widespread Use of Pfizer COVID-19 VaccineA U.S. government advisory panel endorsed widespread use of Pfizer’s coronavirus vaccine Thursday, putting the country just one step away from launching an epic vaccination campaign against the outbreak that has killed close to 300,000 Americans. Shots could begin within days, depending on how quickly the Food and Drug Administration signs off, as expected, on […]
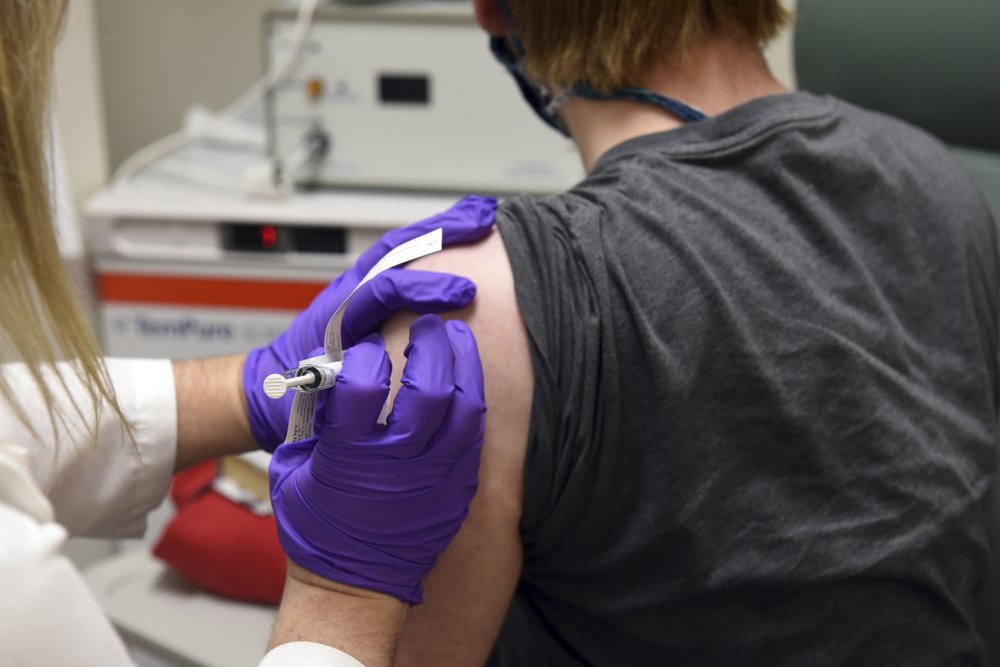
Pfizer COVID-19 Vaccine Faces Last Hurdle Before U.S. DecisionPfizer’s COVID-19 vaccine faces one final hurdle as it races to become the first shot greenlighted in the U.S.: a panel of experts who will scrutinize the company’s data for any red flags. Thursday’s meeting of the Food and Drug Administration’s vaccine advisory panel is likely the last step before a U.S. decision to begin […]
›






