Written by LINDSEY TANNER
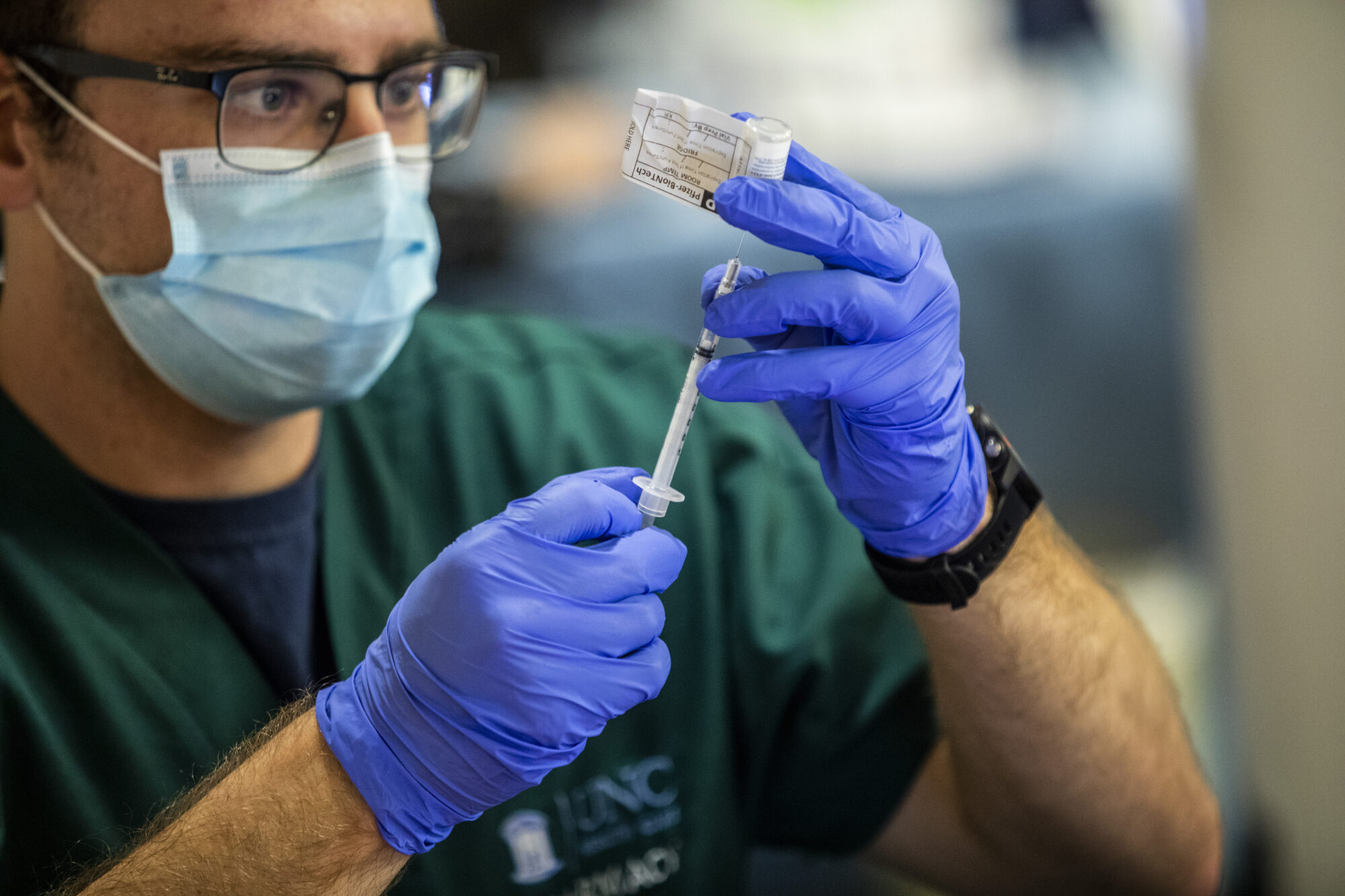
U.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
The Food and Drug Administration’s action follows its advisory panel’s unanimous recommendation for the shots from Moderna and Pfizer. That means U.S. kids under 5 — roughly 18 million youngsters — are eligible for the shots, about 1 1/2 years after the vaccines first became available in the U.S. for adults, who have been hit the hardest during the pandemic.
The FDA also authorized Moderna’s vaccine for school-aged children and teens. Pfizer’s shots had previously been the only ones available for those ages.
There’s one step left: The Centers for Disease Control and Prevention recommends how to use vaccines and its vaccine advisers are set to discuss the shots for the youngest kids Friday and vote on Saturday. A final signoff would come from CDC Director Dr. Rochelle Walensky.
At a Senate hearing Thursday, Walensky said her staff was working over the Juneteenth federal holiday weekend “because we understand the urgency of this for American parents.”
She said pediatric deaths from COVID-19 have been higher than what is generally seen from the flu each year.
“So I actually think we need to protect young children, as well as protect everyone with the vaccine and especially protect elders,” she said.
For weeks, the Biden administration has been preparing to roll out the vaccines. States, tribes, community health centers and pharmacies preordered millions of doses. FDA’s emergency use authorization allows manufacturers to begin shipping vaccine across the country. Vaccinations could begin as early as Monday or Tuesday.
Some parents have been anxiously awaiting the chance to protect their little ones.
While young children generally don’t get as sick from COVID-19 as older kids and adults, their hospitalizations surged during the omicron wave and FDA’s advisers determined that benefits from vaccination outweighed the minimal risks. Studies from Moderna and Pfizer showed side effects, including fever and fatigue, were mostly minor.
The two brands use the same technology but there are differences.
Pfizer’s vaccine for kids younger than 5 is one-tenth of the adult dose. Three shots are needed: the first two given three weeks apart and the last at least two months later.
Moderna’s is two shots, each a quarter of its adult dose, given about four weeks apart for kids under 6.
The vaccines are for children as young as 6 months. Moderna next plans to study its shots for babies as young as 3-months-old. Pfizer has not finalized plans for shots in younger infants. A dozen countries, including China, already vaccinate kids under 5.
Dr. Beth Ebel, professor of pediatrics at University of Washington in Seattle, said the tot-sized vaccines would be especially welcomed by U.S. parents with children in daycare where outbreaks can sideline parents from jobs, adding to financial strain.
“A lot of people are going to be happy and a lot of grandparents are going to be happy, too, because we’ve missed those babies who grew up when you weren’t able to see them,” Ebel said.
Photo via Ben Garver/The Berkshire Eagle and Associated Press.
Related Stories
‹

Kennedy Tries To Defend COVID-19 Vaccine Stance in Raucous Senate HearingU.S. Health Secretary Robert F. Kennedy Jr., facing pointed bipartisan questioning at a rancorous three-hour Senate committee hearing on Thursday, tried to defend his efforts to pull back COVID-19 vaccine recommendations.

CDC Shooting Marks Latest in a String of Hostility Directed at Health Workers. Many Aren’t SurprisedThe attack in Atlanta happened in the face of ongoing public health misinformation and animosity about the safety of immunizations.

UNC Researcher: Overhaul of CDC Vaccine Advisory Group Adds to 'Incredibly Stressful' Health EnvironmentU.S. Health and Human Services Secretary Robert Kennedy Jr. announced one week ago he would shake up one of the key volunteer groups that advises the Centers for Disease Control and federal government on how to use vaccines. All 17 members of the Advisory Committee on Immunization Practices (ACIP), who were approved by President Joe […]
‘Viral Jambalaya’: Early Flu Adding to Woes for US HospitalsWritten by MIKE STOBBE and LINDSEY TANNER As Americans head into the holiday season, a rapidly intensifying flu season is straining hospitals already overburdened with patients sick from other respiratory infections. More than half the states have high or very high levels of flu, unusually high for this early in the season, the government reported Friday. Those […]

US Clears Updated COVID Boosters Targeting Newest VariantsWritten by LAURAN NEERGAARD The U.S. on Wednesday authorized its first update to COVID-19 vaccines, booster doses that target today’s most common omicron strain. Shots could begin within days. The move by the Food and Drug Administration tweaks the recipe of shots made by Pfizer and rival Moderna that already have saved millions of lives. The hope […]
National Expert: Getting Kids Vaccinated Before School is 'Best Thing to Do'Younger children are the age demographic that had the longest waits for COVID-19 vaccines to be approved. So far, their vaccination rates reflect that gap of time, but urgency around getting those initial doses has slowed to a crawl. With a new school year being just around the corner, though, federal health officials are saying […]
Moderna Seeks To Be 1st With COVID Shots for Littlest KidsModerna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
![]()
Armed Gangs Raise Risks in Vaccinating Rural NigeriansWritten by CHINEDU ASADU Yunusa Bawa rolled his motorcycle away from the health care clinic where he works in Kuje, southwest of Nigeria’s capital of Abuja, and secured a black box of COVID-19 vaccine for the rough ride ahead. The rocky and rugged pathway — Bawa described it as a road that “will make you […]
![]()
COVID Vaccine: CDC Expands Booster Rollout, OKs Mixing ShotsWritten by LAURAN NEERGAARD and MIKE STOBBE Millions more Americans can get a COVID-19 booster and choose a different company’s vaccine for that next shot, federal health officials said Thursday. Certain people who received Pfizer vaccinations months ago already are eligible for a booster and now the Centers for Disease Control and Prevention says specific […]
US Regulators Give Full Approval to Pfizer COVID-19 VaccineWritten by LAURAN NEERGAARD and MATTHEW PERRONE The U.S. gave full approval to Pfizer’s COVID-19 vaccine on Monday, a milestone that may help lift public confidence in the shots as the nation battles the most contagious coronavirus mutant yet. The vaccine made by Pfizer and its partner BioNTech now carries the strongest endorsement from the […]
›