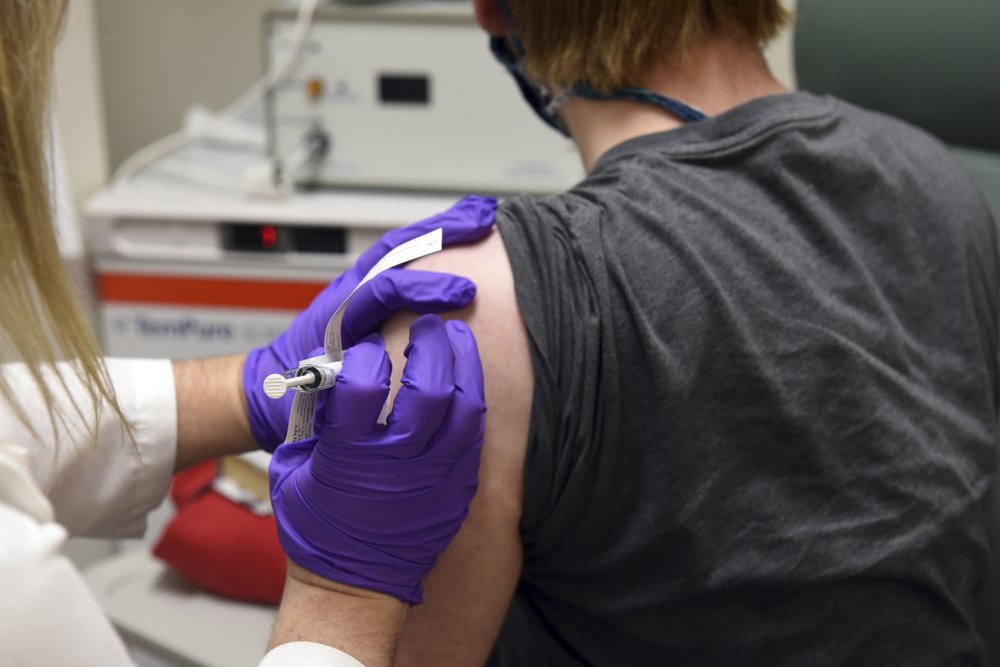
A late-stage study of Johnson & Johnson’s COVID-19 vaccine candidate has been paused while the company investigates whether a study participant’s “unexplained illness” is related to the shot.
The company said in a statement Monday evening that illnesses, accidents and other so-called adverse events “are an expected part of any clinical study, especially large studies,” but that its physicians and a safety monitoring panel would try to determine what might have caused the illness.
The pause is at least the second such hold to occur among several vaccines that have reached large-scale final tests in the U.S.
The company declined to reveal any more details about the illness, citing the participant’s privacy.
Temporary stoppages of large medical studies are relatively common. Few are made public in typical drug trials, but the work to make a coronavirus vaccine has raised the stakes on these kinds of complications.
Companies are required to investigate any serious or unexpected reaction that occurs during drug testing. Given that such tests are done on tens of thousands of people, some medical problems are a coincidence. In fact, one of the first steps the company said it will take is to determine if the person received the vaccine or a placebo.
The halt was first reported by the health news site STAT.
Final-stage testing of a vaccine made by AstraZeneca and Oxford University remains on hold in the U.S. as officials examine whether an illness in its trial poses a safety risk. That trial was stopped when a woman developed severe neurological symptoms consistent with transverse myelitis, a rare inflammation of the spinal cord, the company has said. That company’s testing has restarted elsewhere.
Johnson & Johnson was aiming to enroll 60,000 volunteers to prove if its single-dose approach is safe and protects against the coronavirus. Other vaccine candidates in the U.S. require two shots.
Related Stories
‹
![]()
‘Overwhelm the Problem’: Inside Biden’s War on COVID-19The meetings begin each day not long after dawn. Dozens of aides report in, coffee in hand, joining by Zoom from agency headquarters, their homes or even adjacent offices. The sessions start with the latest sobering statistics meant to focus the work and offer a reminder of what’s at stake: new coronavirus cases, people in […]
![]()
J&J 1-Dose Shot Prevents COVID-19, but Less Than Some OthersJohnson & Johnson’s long-awaited vaccine appears to protect against COVID-19 with just one shot – not as strong as some two-shot rivals but still potentially helpful for a world in dire need of more doses. J&J said Friday that in the U.S. and seven other countries, the single-shot vaccine was 66% effective overall at preventing moderate […]
![]()
3rd Major COVID-19 Vaccine Shown To Be Effective and CheaperPharmaceutical company AstraZeneca said Monday that late-stage trials showed its coronavirus vaccine was up to 90% effective, giving public health officials hope they may soon have access to a vaccine that is cheaper and easier to distribute than some of its rivals. The results are based on interim analysis of trials in the U.K. and […]
Pfizer: COVID-19 Shot 95% Effective, Seeking Clearance SoonPfizer said Wednesday that new test results show its coronavirus vaccine is 95% effective, is safe and also protects older people most at risk of dying — last data needed to seek emergency use of limited shot supplies even as the catastrophic outbreak worsens across the globe. The announcement from Pfizer and its German partner […]
![]()
Biden Seeks Window on Vaccine Plans as Trump Stalls HandoffPresident-elect Joe Biden’s scientific advisers plan to meet with vaccine makers in coming days even as a stalled presidential transition keeps them out of the loop on government plans to inoculate all Americans against COVID-19. President Donald Trump’s refusal to accept that he lost the election means that the Biden team lacks a clear picture of the groundwork within […]

Pfizer Says COVID-19 Vaccine Is Looking 90% EffectivePfizer said Monday that an early peek at the data on its coronavirus vaccine suggests the shots may be a robust 90% effective at preventing COVID-19, putting the company on track to apply later this month for emergency-use approval from the Food and Drug Administration. The announcement, less than a week after a presidential election […]
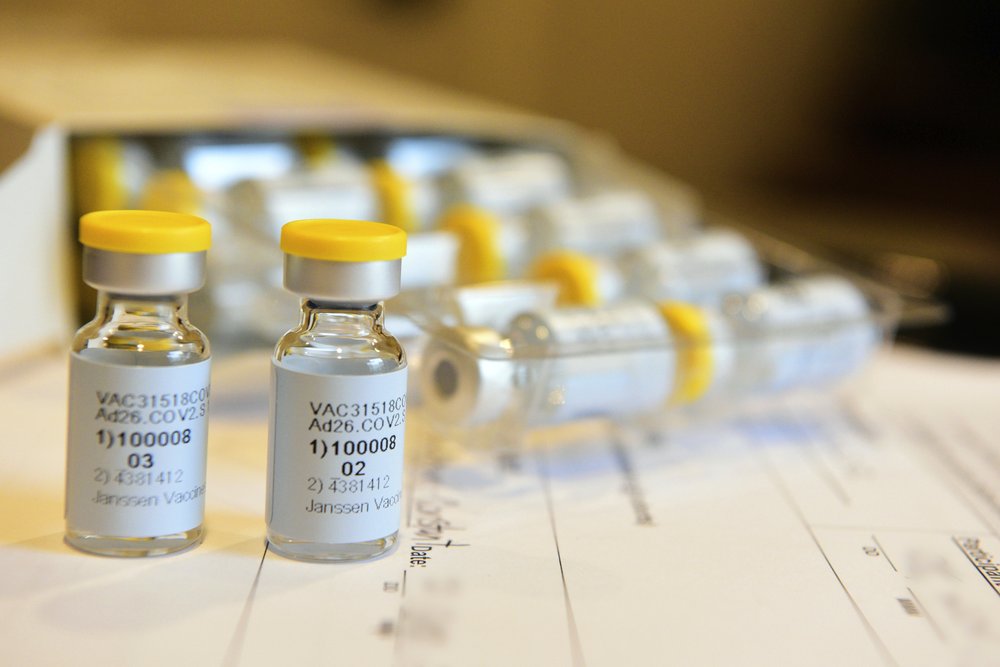
Late-Stage Study of First Single-Shot Vaccine Begins in U.S.Johnson & Johnson is beginning a huge final study to try to prove if a single-dose COVID-19 vaccine can protect against the virus. The study starting Wednesday will be one of the world’s largest coronavirus vaccine studies so far, testing the shot in 60,000 volunteers in the U.S., South Africa, Argentina, Brazil, Chile, Colombia, Mexico […]
![]()
As Rich Nations Struggle, Africa’s Virus Response Is PraisedAt a lecture to peers this month, John Nkengasong showed images that once dogged Africa, with a magazine cover declaring it “The Hopeless Continent.” Then he quoted Ghana’s first president, Kwame Nkrumah: “It is clear that we must find an African solution to our problems, and that this can only be found in African unity.” […]
![]()
U.S. Outlines Sweeping Plan to Provide Free COVID-19 VaccinesThe federal government outlined a sweeping plan Wednesday to make vaccines for COVID-19 available for free to all Americans, even as polls show a strong undercurrent of skepticism rippling across the land. In a report to Congress and an accompanying “playbook” for states and localities, federal health agencies and the Defense Department sketched out complex […]
![]()
Germany Boosts Own Vaccine Makers in Race for COVID-19 JabGermany says it is providing up to 750 million euros to support three domestic pharmaceutical companies that are developing vaccines against the new coronavirus. Science Minister Anja Karliczek said Tuesday that the government has already agreed to provide BioNTech and CureVac with 375 million euros and 230-million euros respectively to develop their mRNA-based vaccines. Talks […]
›



