Written by MATTHEW PERRONE
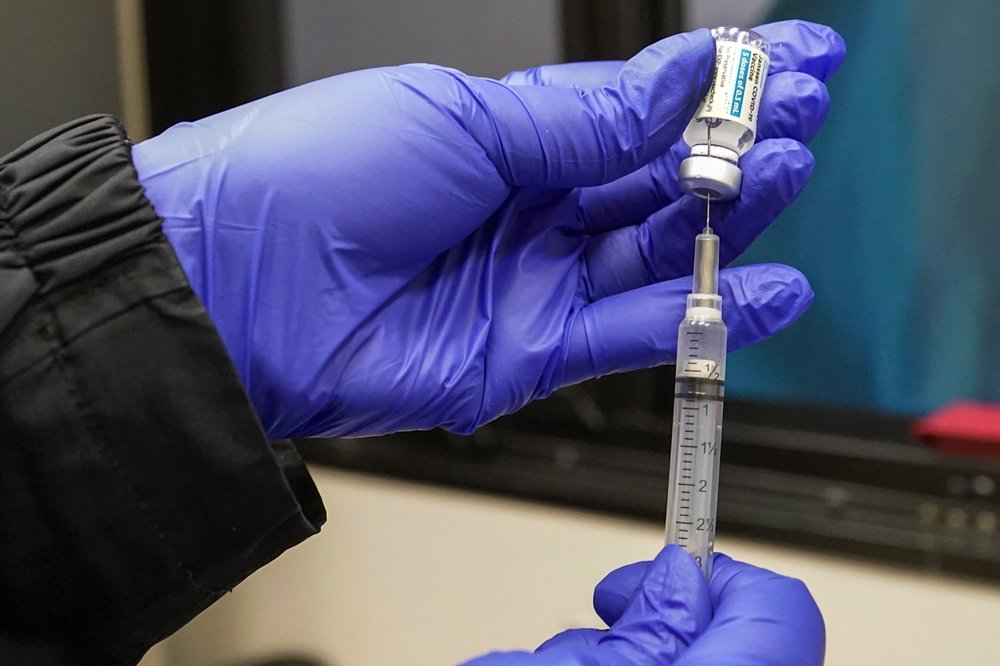
Federal health regulators say an experimental COVID-19 pill from Merck is effective against the virus, but they will seek input from outside experts on risks of birth defects and other potential problems in pregnant women.
The Food and Drug Administration posted its analysis of the pill ahead of a public meeting next week where academic and other experts will weigh in on its safety and effectiveness. The agency isn’t required to follow the group’s advice.
The FDA scientists said their review identified several potential risks, including possible toxicity and birth defects. Regulators also noted Merck collected far less safety data overall on its drug than was gathered for other COVID-19 therapies.
All COVID-19 drugs currently authorized by the FDA require an injection or IV, which limits their use. If authorized, Merck’s drug would be the first that patients could take at home to ease symptoms and speed recovery. It is already authorized for emergency use in the U.K.
With cases rising again across most of the U.S., regulators are expected to greenlight Merck’s drug as an important new weapon to help ease the strain on hospitals. But the FDA review is key to determining who will be eligible and how widely it might be prescribed.
FDA will ask its independent advisers whether the drug’s benefits outweigh its risks and if its use should be restricted for pregnant women.
Related Stories
‹
Here’s Where Jobs and Programs Are Being Cut at the Nation’s Top Health AgenciesThousands of people were laid off Tuesday at the United States' top health agencies. Here are what positions and efforts were cut.

US Clears Updated COVID Boosters Targeting Newest VariantsWritten by LAURAN NEERGAARD The U.S. on Wednesday authorized its first update to COVID-19 vaccines, booster doses that target today’s most common omicron strain. Shots could begin within days. The move by the Food and Drug Administration tweaks the recipe of shots made by Pfizer and rival Moderna that already have saved millions of lives. The hope […]
![]()
Marathon US Hearings To Decide Fate of COVID Shots for TotsWritten by LAURAN NEERGAARD Parents anxious to finally vaccinate their youngest children against COVID-19, strap in: A lot is set to happen over the next week. On Wednesday, both Moderna and Pfizer will have to convince what’s essentially a science court — advisers to the Food and Drug Administration — that their shots work well in babies, toddlers and […]

Baby Formula Shortage Highlights Racial DisparitiesWritten by JACQUELYN MARTIN, ADRIANA GOMEZ LICON and TERRY TANG Capri Isidoro broke down in tears in the office of a lactation consultant. The mother of two had been struggling to breastfeed her 1-month-old daughter ever since she was born, when the hospital gave the baby formula first without consulting her on her desire to […]
![]()
Next Battle Over Access to Abortion Will Focus on PillsWritten by STEPHEN GROVES It took two trips over state lines, navigating icy roads and a patchwork of state laws, for a 32-year-old South Dakota woman to get abortion pills last year. For abortion-seekers like her, such journeys, along with pills sent through the mail, will grow in importance if the Supreme Court follows through […]
![]()
High From Hemp: States Wrestle With Chemically Made THCWritten by GENE JOHNSON Over the past few years, Jonny Griffis has invested millions of dollars in his legal marijuana farm in northern Michigan, which produces extracts to be used in things like gummy bears and vape oils. But now that farm — like many other licensed grows in states that have legalized marijuana — […]

Moderna Says Its Low-Dose COVID Shots Work for Kids Under 6Written by LAURAN NEERGAARD Moderna’s COVID-19 vaccine works in babies, toddlers and preschoolers, the company announced Wednesday — and if regulators agree it could mean a chance to finally start vaccinating the littlest kids by summer. Moderna said in the coming weeks it would ask regulators in the U.S. and Europe to authorize two small-dose […]
![]()
Moderna Seeks FDA Authorization for 4th Dose of COVID ShotWritten by ZEKE MILLER Drugmaker Moderna asked the Food and Drug Administration on Thursday to authorize a fourth shot of its COVID-19 vaccine as a booster dose for all adults. The request is broader than rival pharmaceutical company Pfizer’s request earlier this week for the regulator to approve a booster shot for all seniors. In […]
![]()
After SCOTUS Hearing, a New Look at Baby ‘Safe Haven’ LawsWritten by ASTRID GALVAN For years, Nicole Olson had longed for a baby and gone through a rigorous and emotional adoption process. Then Olson and her husband got a call asking if they’d like to adopt a newborn. That day. As soon as possible. The baby had been relinquished through what’s known as a safe […]
![]()
FDA: Merck COVID Pill Effective, Experts Will Review SafetyWritten by MATTHEW PERRONE Federal health regulators say an experimental COVID-19 pill from Merck is effective against the virus, but they will seek input from outside experts on risks of birth defects and other potential problems in pregnant women. The Food and Drug Administration posted its analysis of the pill ahead of a public meeting […]
›





